|
|
|
Eat fiber! A diet high in fiber can help lower cholesterol levels by as much as 10%.
Medication errors are three times higher among children and infants than with adults.
The average person is easily confused by the terms pharmaceutics and pharmacology, thinking they are one and the same. Whereas pharmaceutics is the science of preparing and dispensing drugs (otherwise known as the science of pharmacy), pharmacology is the study of medications.
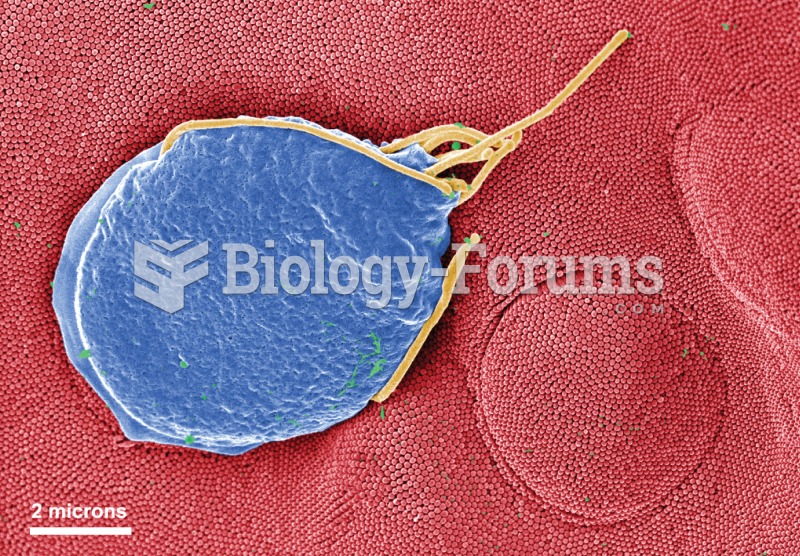
About one in five American adults and teenagers have had a genital herpes infection—and most of them don't know it. People with genital herpes have at least twice the risk of becoming infected with HIV if exposed to it than those people who do not have genital herpes.
The average adult has about 21 square feet of skin.