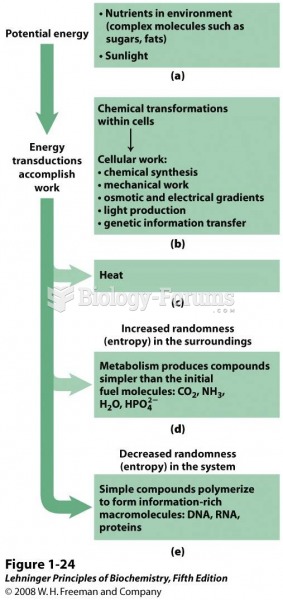
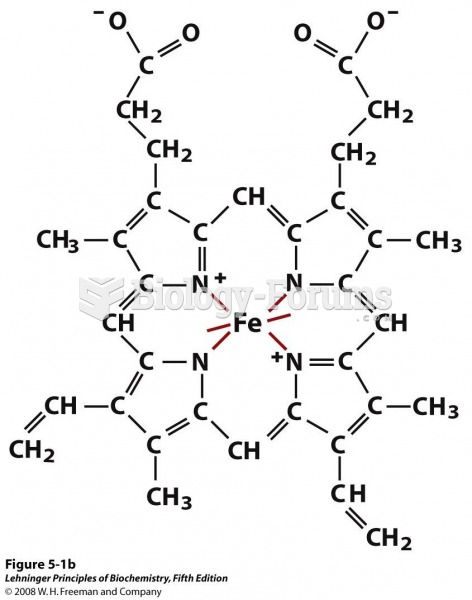
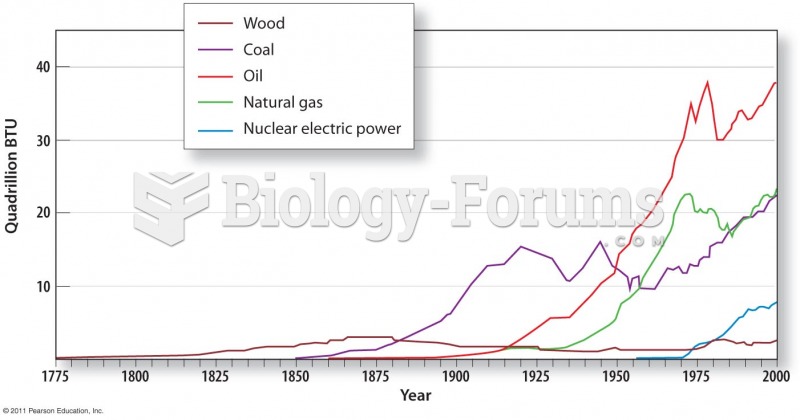
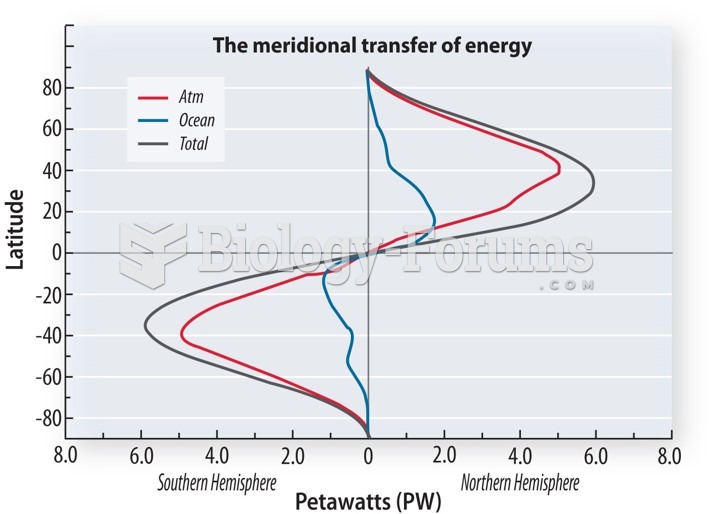
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
There are more sensory neurons in the tongue than in any other part of the body.
Did you know?
In women, pharmacodynamic differences include increased sensitivity to (and increased effectiveness of) beta-blockers, opioids, selective serotonin reuptake inhibitors, and typical antipsychotics.
Did you know?
Human kidneys will clean about 1 million gallons of blood in an average lifetime.
Did you know?
Vampire bats have a natural anticoagulant in their saliva that permits continuous bleeding after they painlessly open a wound with their incisors. This capillary blood does not cause any significant blood loss to their victims.
Did you know?
More than 34,000 trademarked medication names and more than 10,000 generic medication names are in use in the United States.