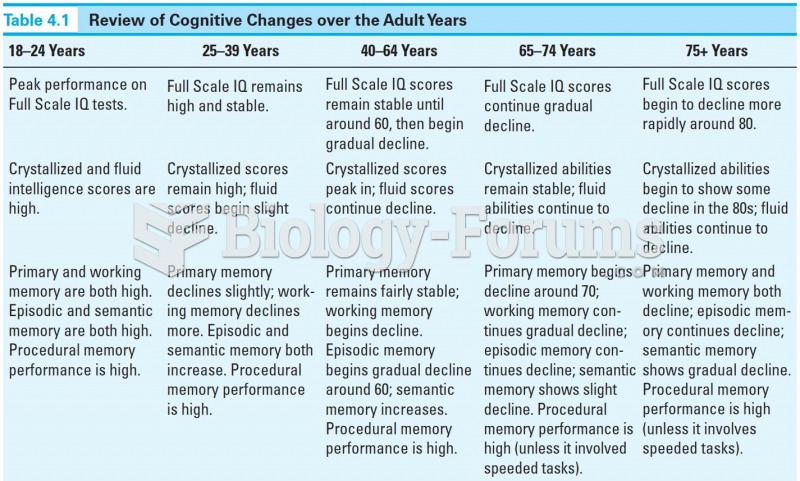
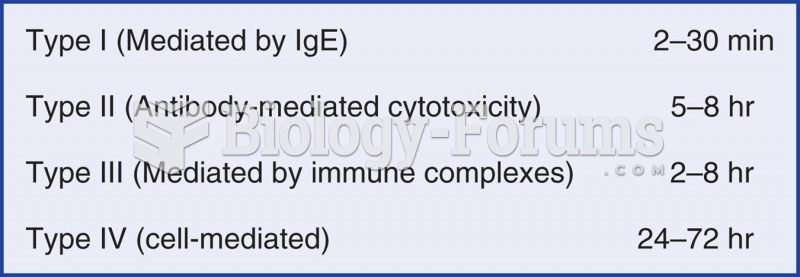
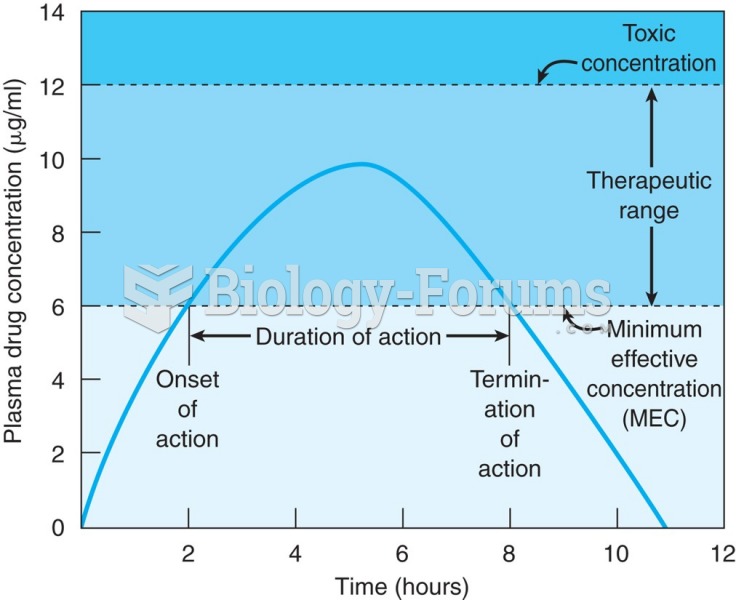
|
|
|
Addicts to opiates often avoid treatment because they are afraid of withdrawal. Though unpleasant, with proper management, withdrawal is rarely fatal and passes relatively quickly.
Aspirin may benefit 11 different cancers, including those of the colon, pancreas, lungs, prostate, breasts, and leukemia.
There are over 65,000 known species of protozoa. About 10,000 species are parasitic.
The top 10 most important tips that will help you grow old gracefully include (1) quit smoking, (2) keep your weight down, (3) take supplements, (4) skip a meal each day or fast 1 day per week, (5) get a pet, (6) get medical help for chronic pain, (7) walk regularly, (8) reduce arguments, (9) put live plants in your living space, and (10) do some weight training.
On average, someone in the United States has a stroke about every 40 seconds. This is about 795,000 people per year.
 Older adults may grieve intensely over the loss of a person or situation that has been a part of the
Older adults may grieve intensely over the loss of a person or situation that has been a part of the
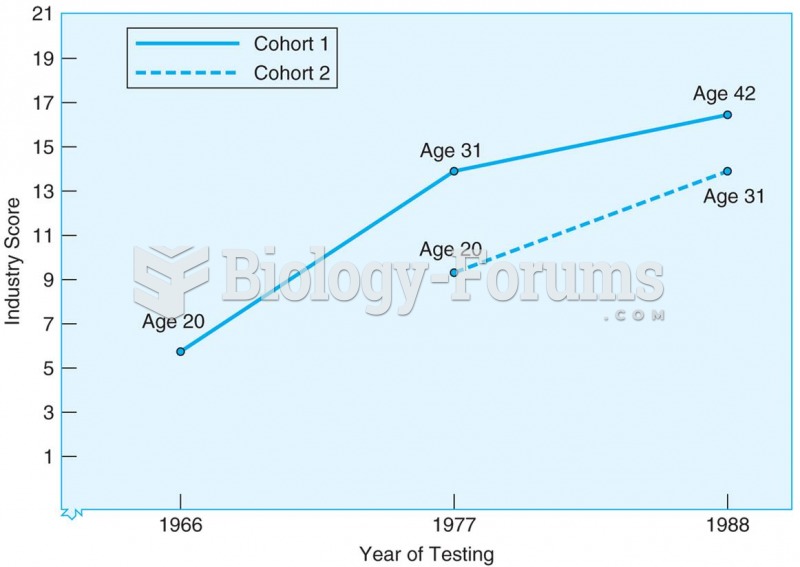 Results from sequential study of two cohorts tested at three ages and at three different points in t
Results from sequential study of two cohorts tested at three ages and at three different points in t