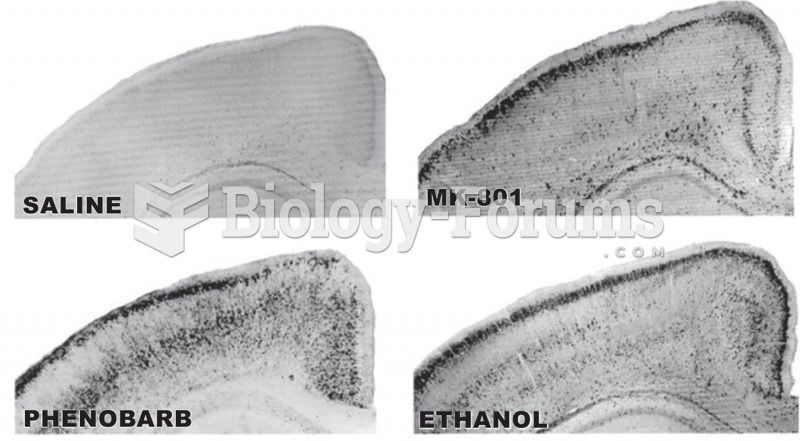
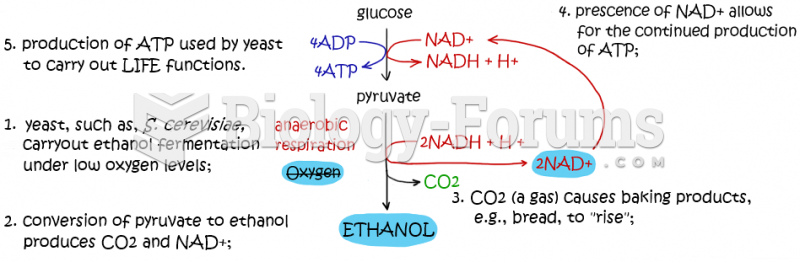
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Asthma cases in Americans are about 75% higher today than they were in 1980.
Did you know?
Everyone has one nostril that is larger than the other.
Did you know?
Cyanide works by making the human body unable to use oxygen.
Did you know?
Pregnant women usually experience a heightened sense of smell beginning late in the first trimester. Some experts call this the body's way of protecting a pregnant woman from foods that are unsafe for the fetus.
Did you know?
One way to reduce acid reflux is to lose two or three pounds. Most people lose weight in the belly area first when they increase exercise, meaning that heartburn can be reduced quickly by this method.