|
|
|
Drug abusers experience the following scenario: The pleasure given by their drug (or drugs) of choice is so strong that it is difficult to eradicate even after years of staying away from the substances involved. Certain triggers may cause a drug abuser to relapse. Research shows that long-term drug abuse results in significant changes in brain function that persist long after an individual stops using drugs. It is most important to realize that the same is true of not just illegal substances but alcohol and tobacco as well.
Chronic necrotizing aspergillosis has a slowly progressive process that, unlike invasive aspergillosis, does not spread to other organ systems or the blood vessels. It most often affects middle-aged and elderly individuals, spreading to surrounding tissue in the lungs. The disease often does not respond to conventionally successful treatments, and requires individualized therapies in order to keep it from becoming life-threatening.
Drying your hands with a paper towel will reduce the bacterial count on your hands by 45–60%.
During pregnancy, a woman is more likely to experience bleeding gums and nosebleeds caused by hormonal changes that increase blood flow to the mouth and nose.
Barbituric acid, the base material of barbiturates, was first synthesized in 1863 by Adolph von Bayer. His company later went on to synthesize aspirin for the first time, and Bayer aspirin is still a popular brand today.
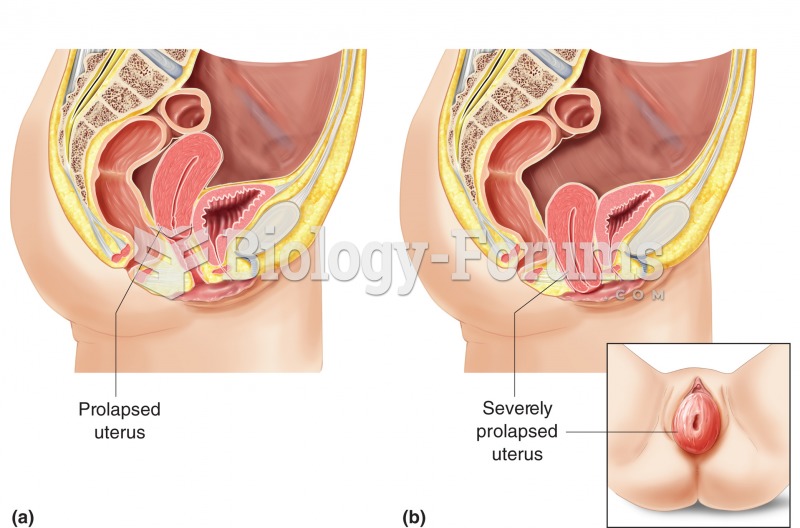 Prolapsed uterus. (a) A prolapse is the abnormal drop of the uterus into the vagina, representing th
Prolapsed uterus. (a) A prolapse is the abnormal drop of the uterus into the vagina, representing th
 A the subfossil lemurs of Madagascar filled a variety of niches occupied elsewhere by monkeys, as sh
A the subfossil lemurs of Madagascar filled a variety of niches occupied elsewhere by monkeys, as sh
 As the glass ceiling slowly cracks, women are gaining entry into the top positions of society. Shown ...
As the glass ceiling slowly cracks, women are gaining entry into the top positions of society. Shown ...