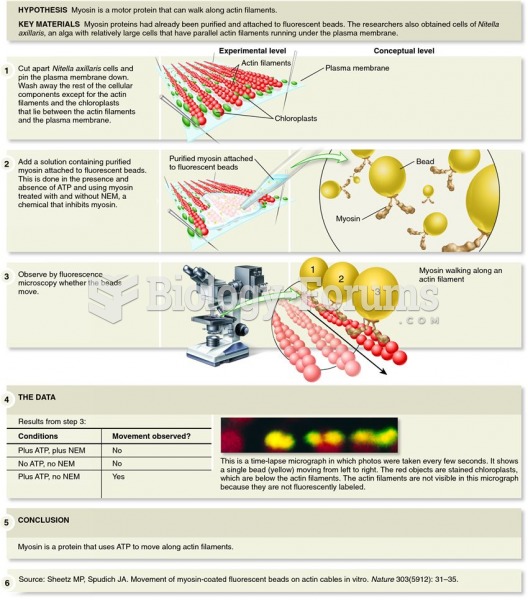
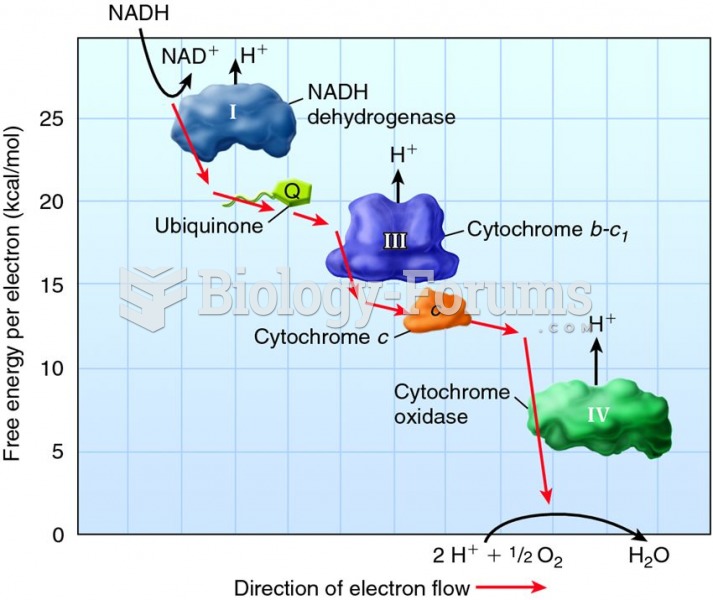
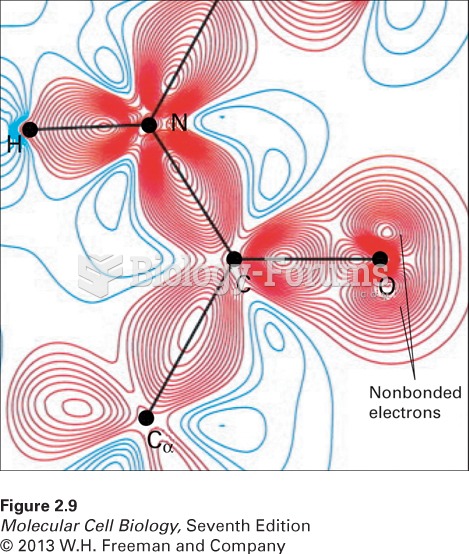
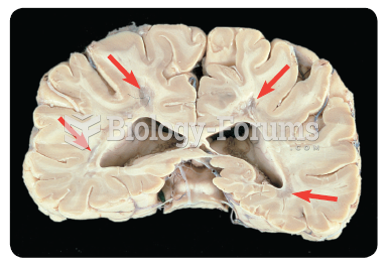
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Methicillin-resistant Staphylococcus aureus or MRSA was discovered in 1961 in the United Kingdom. It if often referred to as a superbug. MRSA infections cause more deaths in the United States every year than AIDS.
Did you know?
The FDA recognizes 118 routes of administration.
Did you know?
About 3.2 billion people, nearly half the world population, are at risk for malaria. In 2015, there are about 214 million malaria cases and an estimated 438,000 malaria deaths.
Did you know?
Bacteria have flourished on the earth for over three billion years. They were the first life forms on the planet.
Did you know?
Approximately 500,000 babies are born each year in the United States to teenage mothers.