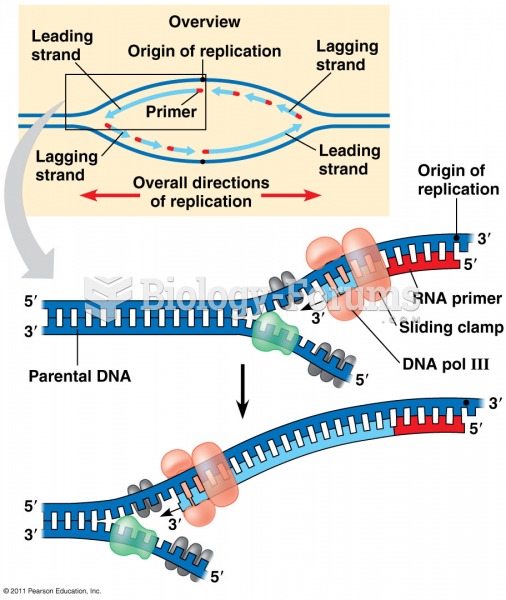
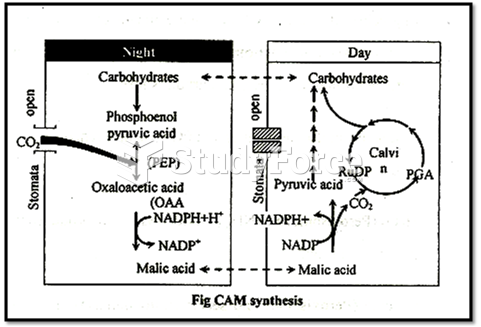
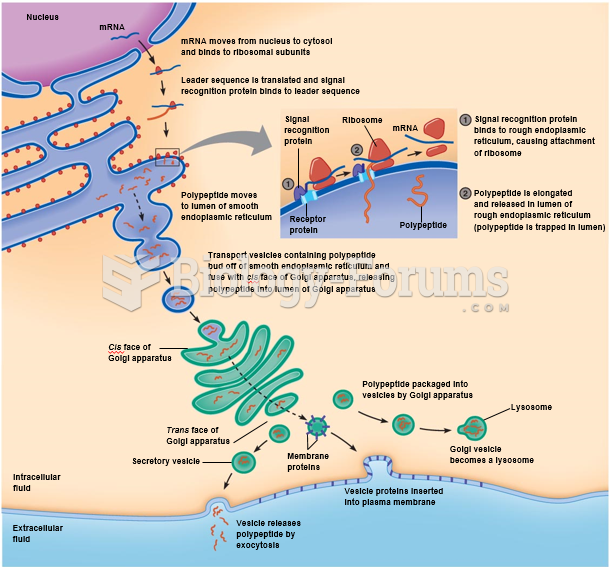
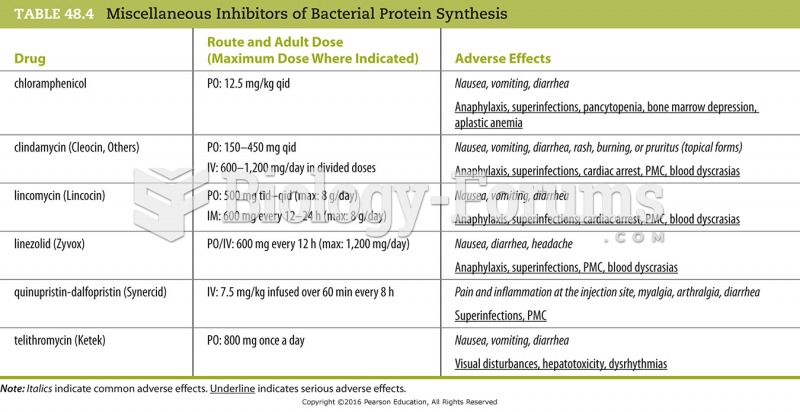
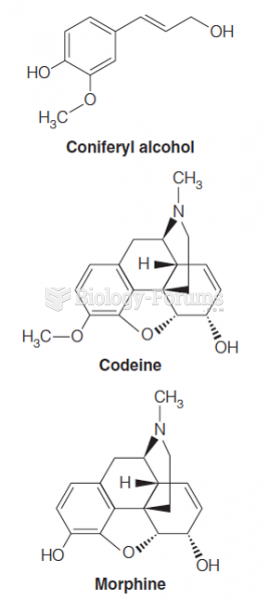
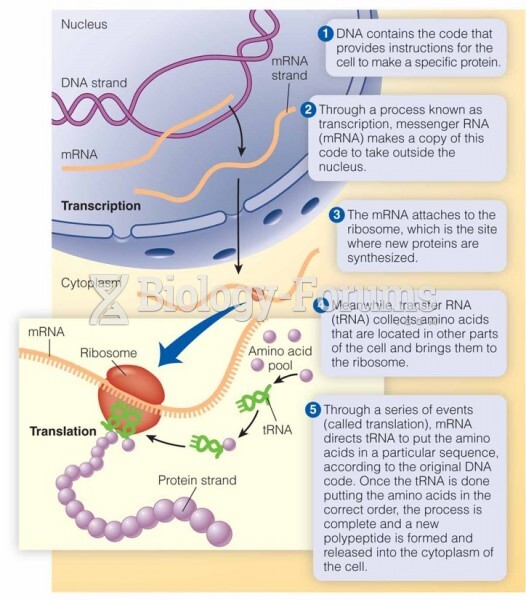
|
|
|
Methicillin-resistant Staphylococcus aureus or MRSA was discovered in 1961 in the United Kingdom. It if often referred to as a superbug. MRSA infections cause more deaths in the United States every year than AIDS.
In the United States, there is a birth every 8 seconds, according to the U.S. Census Bureau's Population Clock.
Pubic lice (crabs) are usually spread through sexual contact. You cannot catch them by using a public toilet.
The first successful kidney transplant was performed in 1954 and occurred in Boston. A kidney from an identical twin was transplanted into his dying brother's body and was not rejected because it did not appear foreign to his body.
The most destructive flu epidemic of all times in recorded history occurred in 1918, with approximately 20 million deaths worldwide.