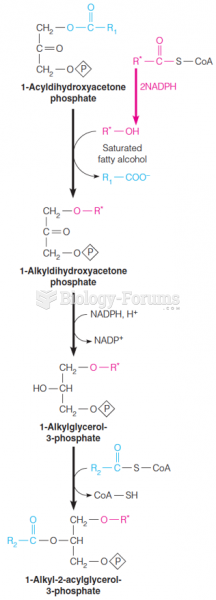
|
|
|
Medication errors are more common among seriously ill patients than with those with minor conditions.
Drug abusers experience the following scenario: The pleasure given by their drug (or drugs) of choice is so strong that it is difficult to eradicate even after years of staying away from the substances involved. Certain triggers may cause a drug abuser to relapse. Research shows that long-term drug abuse results in significant changes in brain function that persist long after an individual stops using drugs. It is most important to realize that the same is true of not just illegal substances but alcohol and tobacco as well.
Certain rare plants containing cyanide include apricot pits and a type of potato called cassava. Fortunately, only chronic or massive ingestion of any of these plants can lead to serious poisoning.
Patients who have been on total parenteral nutrition for more than a few days may need to have foods gradually reintroduced to give the digestive tract time to start working again.
Egg cells are about the size of a grain of sand. They are formed inside of a female's ovaries before she is even born.
 One danger of a wire fence is that, as shown in this photo, it is practically invisible; a running a
One danger of a wire fence is that, as shown in this photo, it is practically invisible; a running a
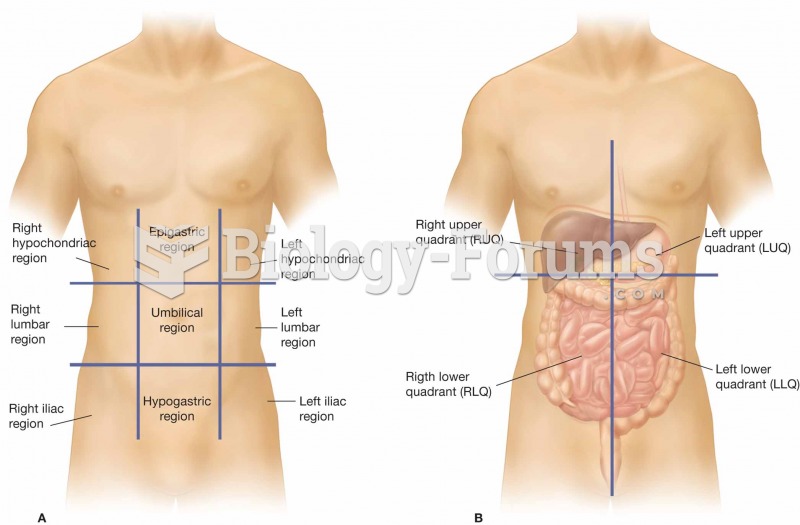 (A) The nine regions of the abdominopelvic cavity. (B) The four regions of the abdomen, which are re
(A) The nine regions of the abdominopelvic cavity. (B) The four regions of the abdomen, which are re
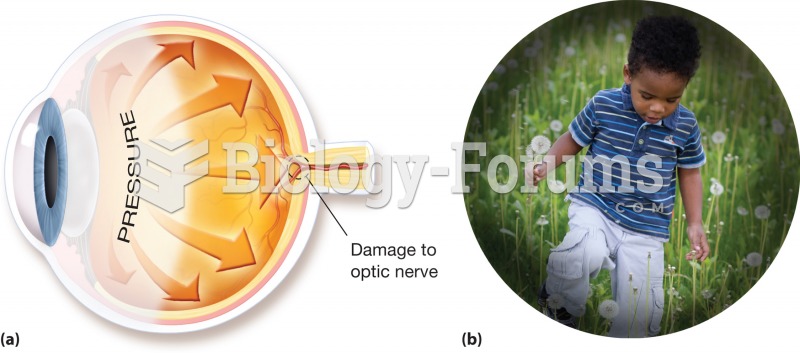 Glaucoma. (a) A buildup of pressure within the eye cavities, often caused by a blockage of vessels t
Glaucoma. (a) A buildup of pressure within the eye cavities, often caused by a blockage of vessels t