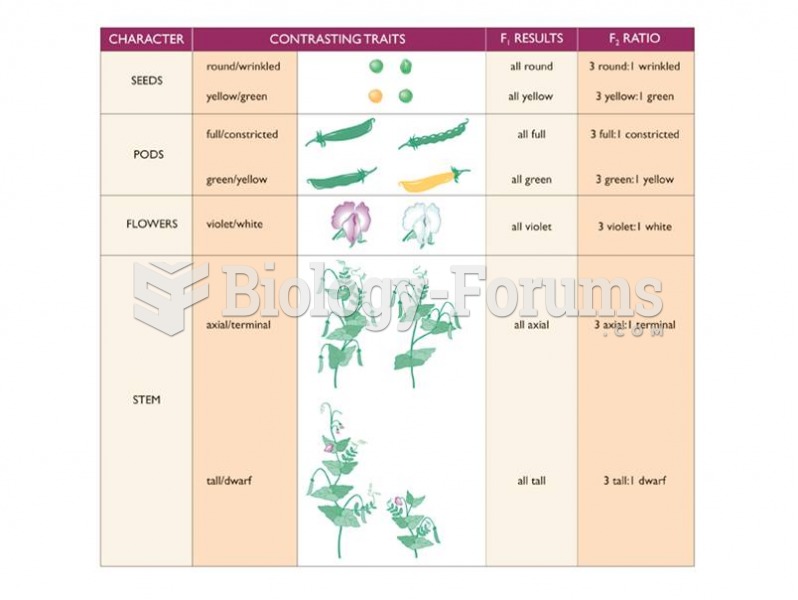
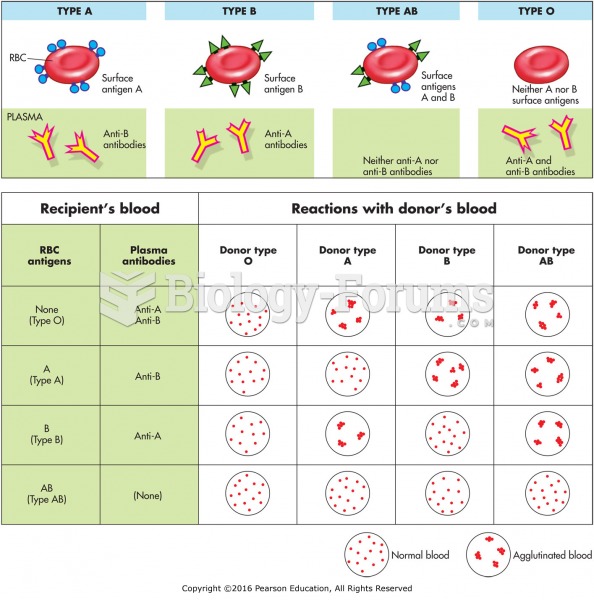
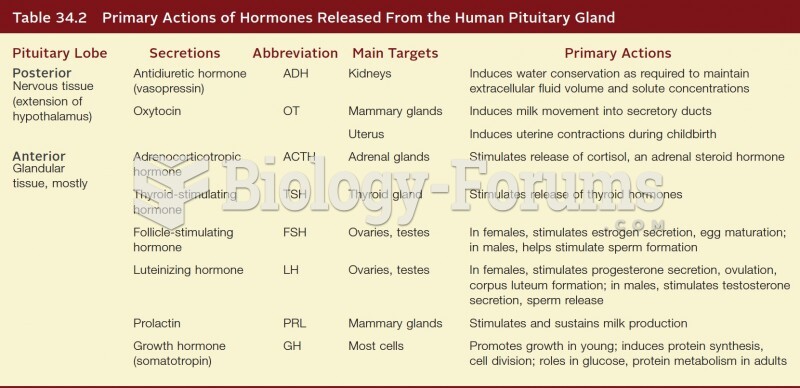
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Allergies play a major part in the health of children. The most prevalent childhood allergies are milk, egg, soy, wheat, peanuts, tree nuts, and seafood.
Did you know?
The most common treatment options for addiction include psychotherapy, support groups, and individual counseling.
Did you know?
Interferon was scarce and expensive until 1980, when the interferon gene was inserted into bacteria using recombinant DNA technology, allowing for mass cultivation and purification from bacterial cultures.
Did you know?
After 5 years of being diagnosed with rheumatoid arthritis, one every three patients will no longer be able to work.
Did you know?
The ratio of hydrogen atoms to oxygen in water (H2O) is 2:1.