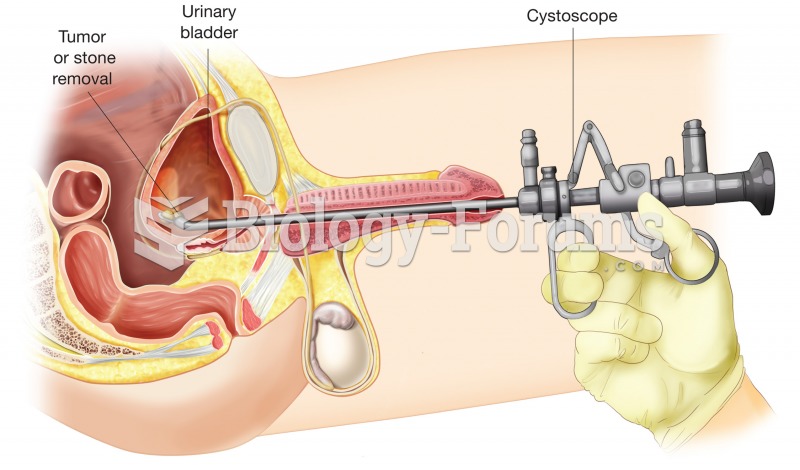
|
|
|
About one in five American adults and teenagers have had a genital herpes infection—and most of them don't know it. People with genital herpes have at least twice the risk of becoming infected with HIV if exposed to it than those people who do not have genital herpes.
Oliver Wendell Holmes is credited with introducing the words "anesthesia" and "anesthetic" into the English language in 1846.
The most destructive flu epidemic of all times in recorded history occurred in 1918, with approximately 20 million deaths worldwide.
The cure for trichomoniasis is easy as long as the patient does not drink alcoholic beverages for 24 hours. Just a single dose of medication is needed to rid the body of the disease. However, without proper precautions, an individual may contract the disease repeatedly. In fact, most people develop trichomoniasis again within three months of their last treatment.
The eye muscles are the most active muscles in the whole body. The external muscles that move the eyes are the strongest muscles in the human body for the job they have to do. They are 100 times more powerful than they need to be.
 A the subfossil lemurs of Madagascar filled a variety of niches occupied elsewhere by monkeys, as sh
A the subfossil lemurs of Madagascar filled a variety of niches occupied elsewhere by monkeys, as sh
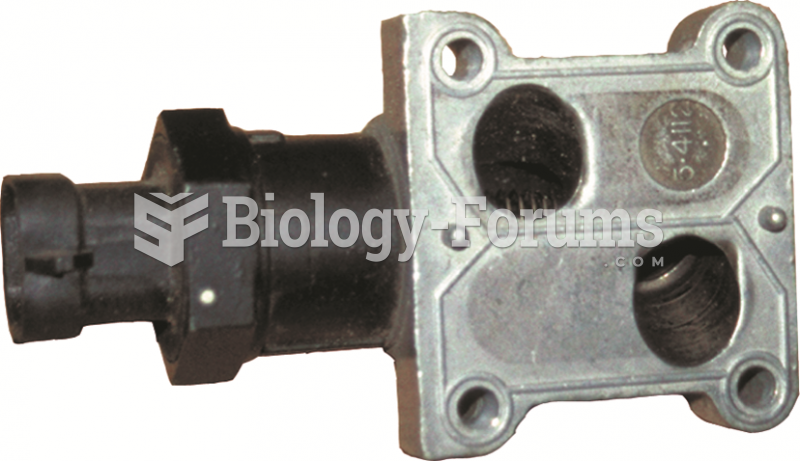 Some IAC units are purchased with the housing as shown. Carbon buildup in these passages can cause ...
Some IAC units are purchased with the housing as shown. Carbon buildup in these passages can cause ...