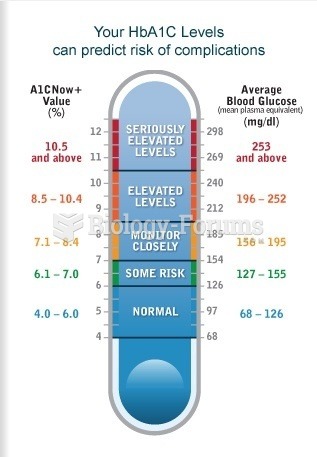
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
There are approximately 3 million unintended pregnancies in the United States each year.
Did you know?
Individuals are never “cured” of addictions. Instead, they learn how to manage their disease to lead healthy, balanced lives.
Did you know?
Only 12 hours after an egg cell is fertilized by a sperm cell, the egg cell starts to divide. As it continues to divide, it moves along the fallopian tube toward the uterus at about 1 inch per day.
Did you know?
In 2012, nearly 24 milliion Americans, aged 12 and older, had abused an illicit drug, according to the National Institute on Drug Abuse (NIDA).
Did you know?
Today, nearly 8 out of 10 pregnant women living with HIV (about 1.1 million), receive antiretrovirals.