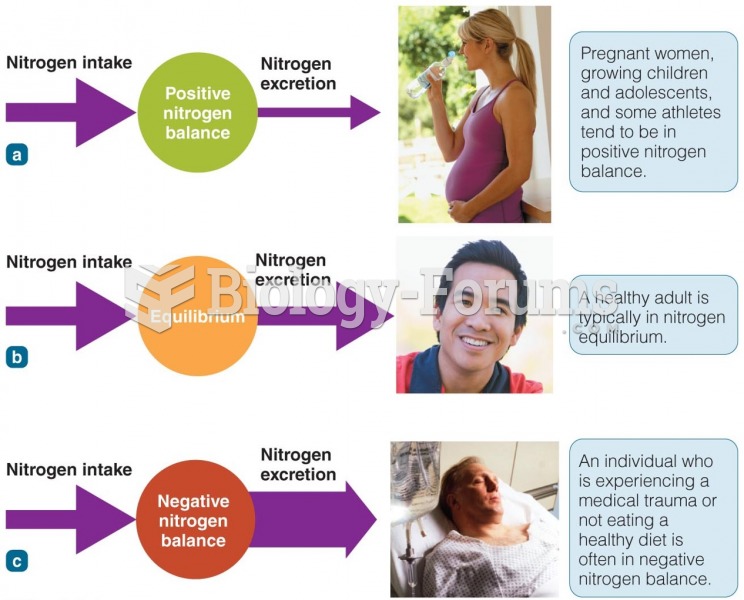
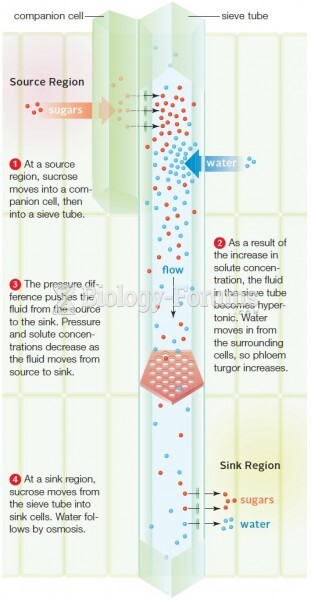
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The calories found in one piece of cherry cheesecake could light a 60-watt light bulb for 1.5 hours.
Did you know?
There are more nerve cells in one human brain than there are stars in the Milky Way.
Did you know?
About 100 new prescription or over-the-counter drugs come into the U.S. market every year.
Did you know?
Fewer than 10% of babies are born on their exact due dates, 50% are born within 1 week of the due date, and 90% are born within 2 weeks of the date.
Did you know?
Nearly all drugs pass into human breast milk. How often a drug is taken influences the amount of drug that will pass into the milk. Medications taken 30 to 60 minutes before breastfeeding are likely to be at peak blood levels when the baby is nursing.