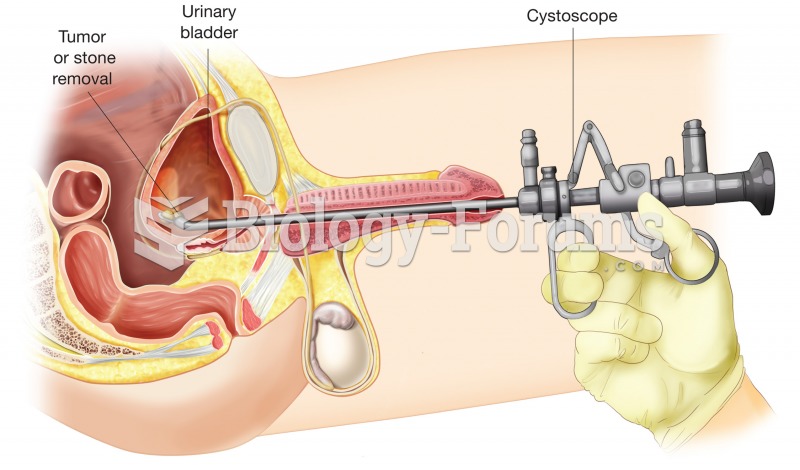
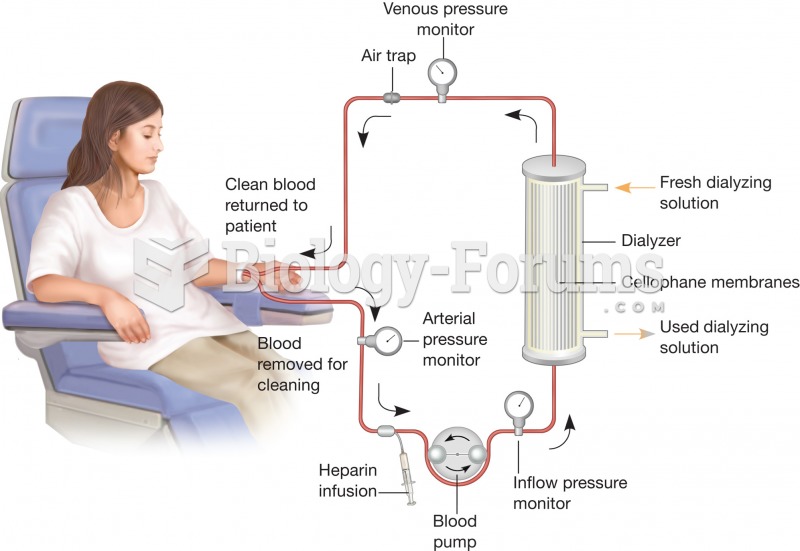
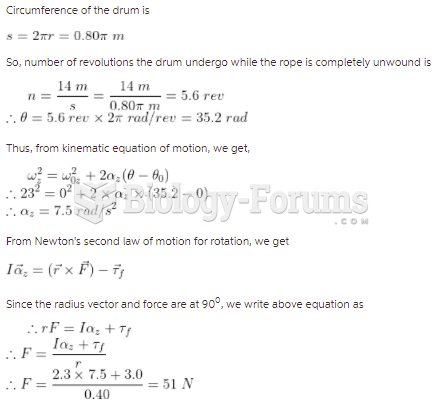
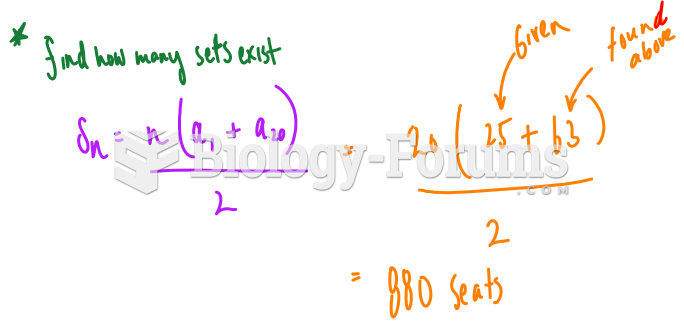This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Human neurons are so small that they require a microscope in order to be seen. However, some neurons can be up to 3 feet long, such as those that extend from the spinal cord to the toes.
Did you know?
Asthma attacks and symptoms usually get started by specific triggers (such as viruses, allergies, gases, and air particles). You should talk to your doctor about these triggers and find ways to avoid or get rid of them.
Did you know?
About 3.2 billion people, nearly half the world population, are at risk for malaria. In 2015, there are about 214 million malaria cases and an estimated 438,000 malaria deaths.
Did you know?
Illicit drug use costs the United States approximately $181 billion every year.
Did you know?
There are more nerve cells in one human brain than there are stars in the Milky Way.