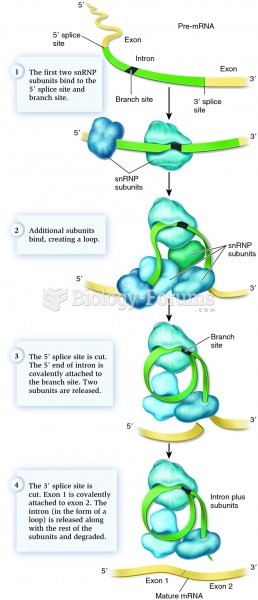
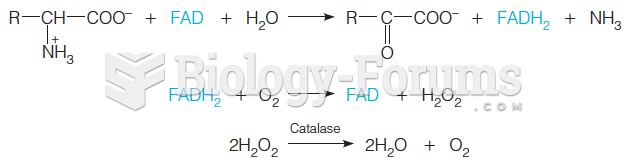
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Adult head lice are gray, about ? inch long, and often have a tiny dot on their backs. A female can lay between 50 and 150 eggs within the several weeks that she is alive. They feed on human blood.
Did you know?
The human body produces and destroys 15 million blood cells every second.
Did you know?
Cyanide works by making the human body unable to use oxygen.
Did you know?
There are actually 60 minerals, 16 vitamins, 12 essential amino acids, and three essential fatty acids that your body needs every day.
Did you know?
Oliver Wendell Holmes is credited with introducing the words "anesthesia" and "anesthetic" into the English language in 1846.
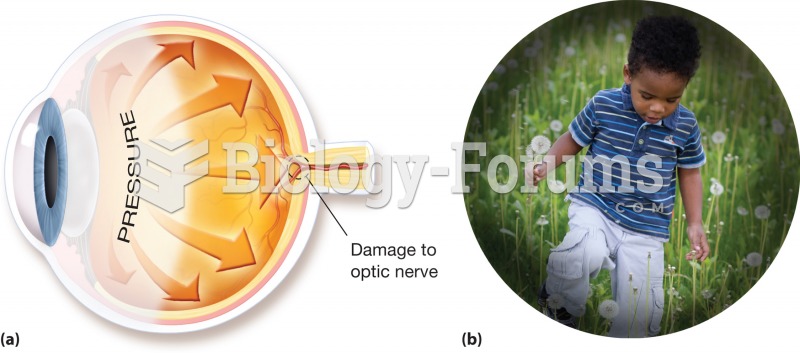 Glaucoma. (a) A buildup of pressure within the eye cavities, often caused by a blockage of vessels t
Glaucoma. (a) A buildup of pressure within the eye cavities, often caused by a blockage of vessels t
 Record the AC frequency as shown on the meter and subtract 50 from the reading (e.g., 60.50 -50.00 = ...
Record the AC frequency as shown on the meter and subtract 50 from the reading (e.g., 60.50 -50.00 = ...