|
|
|
About 80% of major fungal systemic infections are due to Candida albicans. Another form, Candida peritonitis, occurs most often in postoperative patients. A rare disease, Candida meningitis, may follow leukemia, kidney transplant, other immunosuppressed factors, or when suffering from Candida septicemia.
Each year in the United States, there are approximately six million pregnancies. This means that at any one time, about 4% of women in the United States are pregnant.
Approximately one in four people diagnosed with diabetes will develop foot problems. Of these, about one-third will require lower extremity amputation.
Nearly all drugs pass into human breast milk. How often a drug is taken influences the amount of drug that will pass into the milk. Medications taken 30 to 60 minutes before breastfeeding are likely to be at peak blood levels when the baby is nursing.
Urine turns bright yellow if larger than normal amounts of certain substances are consumed; one of these substances is asparagus.
 Scaling the Earth down to the size of a basketball, the Moon is roughly the size of a tennis ball. T
Scaling the Earth down to the size of a basketball, the Moon is roughly the size of a tennis ball. T
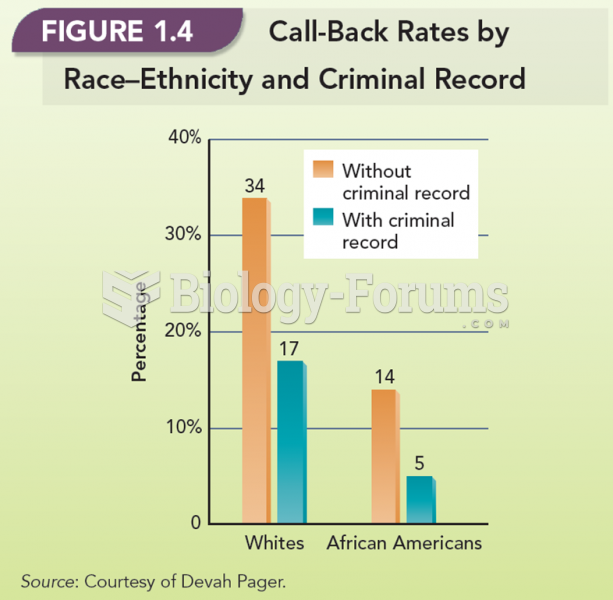 In a project on job discrimination, Pager prepared identical résumés for the teams, but with one ...
In a project on job discrimination, Pager prepared identical résumés for the teams, but with one ...