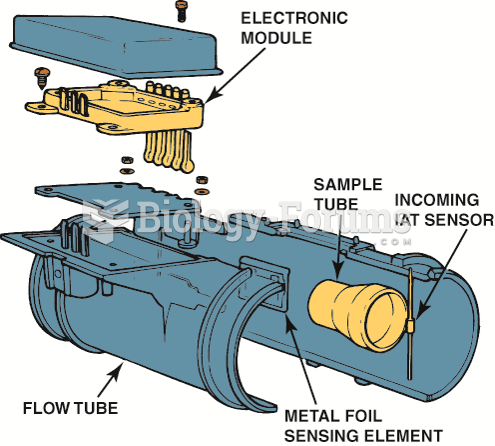
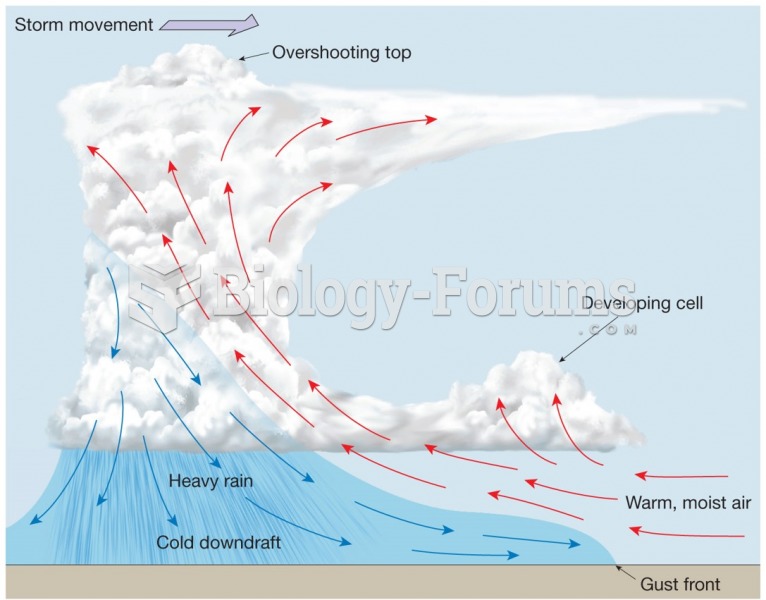
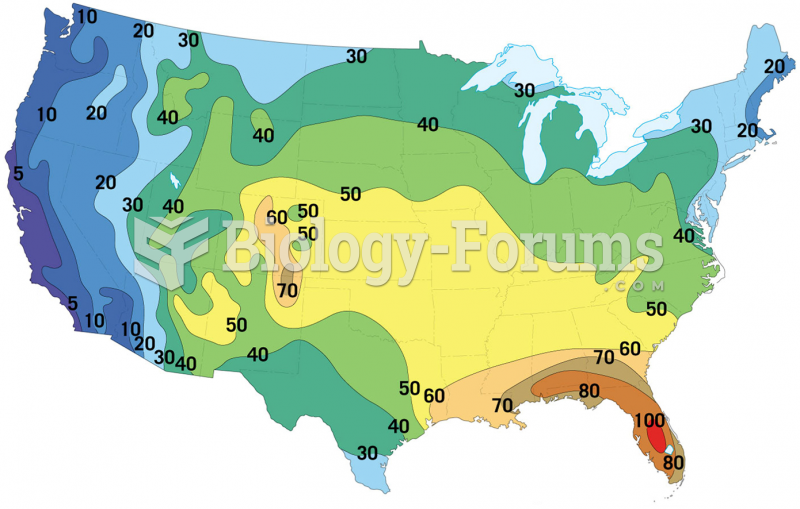
|
|
|
The top five reasons that children stay home from school are as follows: colds, stomach flu (gastroenteritis), ear infection (otitis media), pink eye (conjunctivitis), and sore throat.
Coca-Cola originally used coca leaves and caffeine from the African kola nut. It was advertised as a therapeutic agent and "pickerupper." Eventually, its formulation was changed, and the coca leaves were removed because of the effects of regulation on cocaine-related products.
Human stomach acid is strong enough to dissolve small pieces of metal such as razor blades or staples.
Nearly 31 million adults in America have a total cholesterol level that is more than 240 mg per dL.
In the United States, an estimated 50 million unnecessary antibiotics are prescribed for viral respiratory infections.