|
|
|
Asthma cases in Americans are about 75% higher today than they were in 1980.
Side effects from substance abuse include nausea, dehydration, reduced productivitiy, and dependence. Though these effects usually worsen over time, the constant need for the substance often overcomes rational thinking.
The first war in which wide-scale use of anesthetics occurred was the Civil War, and 80% of all wounds were in the extremities.
The liver is the only organ that has the ability to regenerate itself after certain types of damage. As much as 25% of the liver can be removed, and it will still regenerate back to its original shape and size. However, the liver cannot regenerate after severe damage caused by alcohol.
Today, nearly 8 out of 10 pregnant women living with HIV (about 1.1 million), receive antiretrovirals.
 Cade and colleagues discovered a way to improve athletic performance and prevent salt and water imba
Cade and colleagues discovered a way to improve athletic performance and prevent salt and water imba
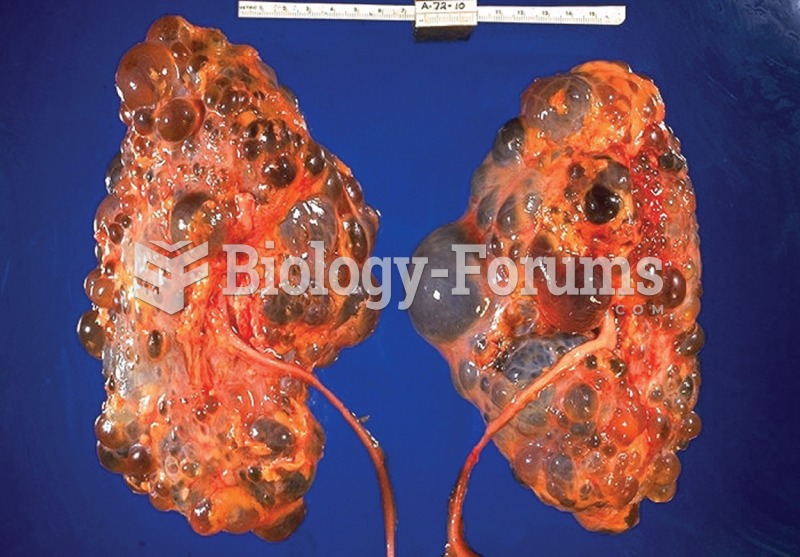
 Prostate cancer. In this example, a large mass has grown into the urinary bladder. Prostate cancer i
Prostate cancer. In this example, a large mass has grown into the urinary bladder. Prostate cancer i