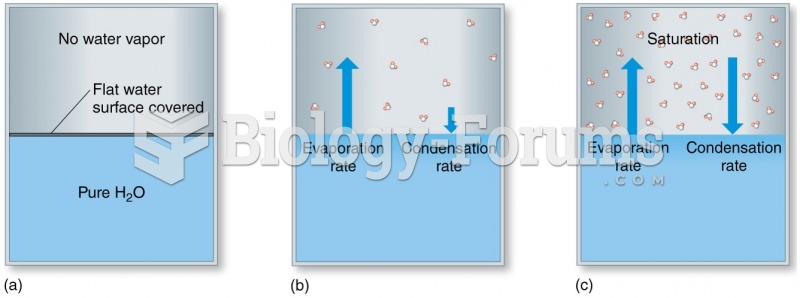
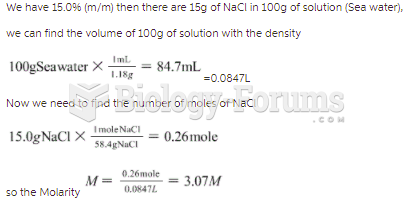|
|
|
More than nineteen million Americans carry the factor V gene that causes blood clots, pulmonary embolism, and heart disease.
The National Institutes of Health have supported research into acupuncture. This has shown that acupuncture significantly reduced pain associated with osteoarthritis of the knee, when used as a complement to conventional therapies.
Computer programs are available that crosscheck a new drug's possible trade name with all other trade names currently available. These programs detect dangerous similarities between names and alert the manufacturer of the drug.
Your chance of developing a kidney stone is 1 in 10. In recent years, approximately 3.7 million people in the United States were diagnosed with a kidney disease.
The training of an anesthesiologist typically requires four years of college, 4 years of medical school, 1 year of internship, and 3 years of residency.
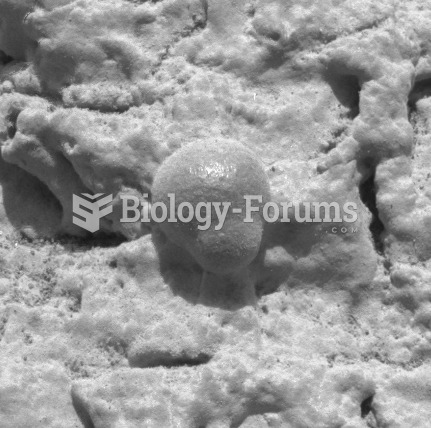 Microscopic photo taken by Opportunity showing a gray hematite concretion, indicative of the past pr
Microscopic photo taken by Opportunity showing a gray hematite concretion, indicative of the past pr
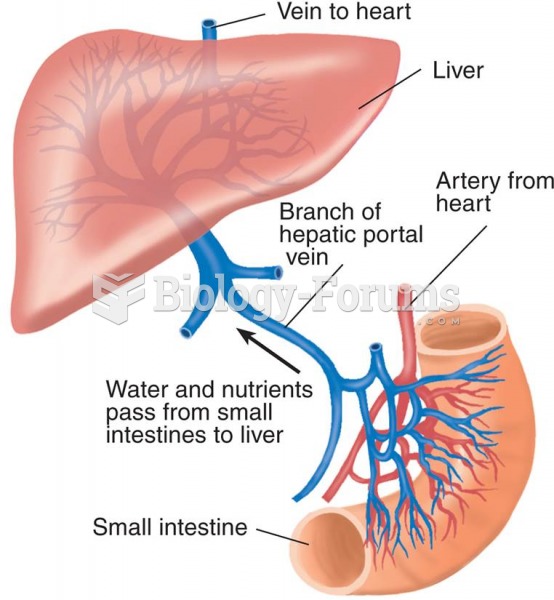 The Hepatic Portal Blood Supply The liver receives water, minerals, and nutrients from the digestive
The Hepatic Portal Blood Supply The liver receives water, minerals, and nutrients from the digestive