This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The most common childhood diseases include croup, chickenpox, ear infections, flu, pneumonia, ringworm, respiratory syncytial virus, scabies, head lice, and asthma.
Did you know?
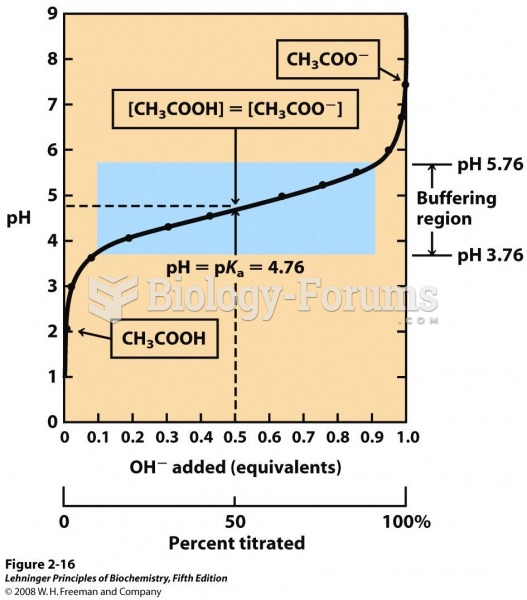
Approximately 25% of all reported medication errors result from some kind of name confusion.
Did you know?
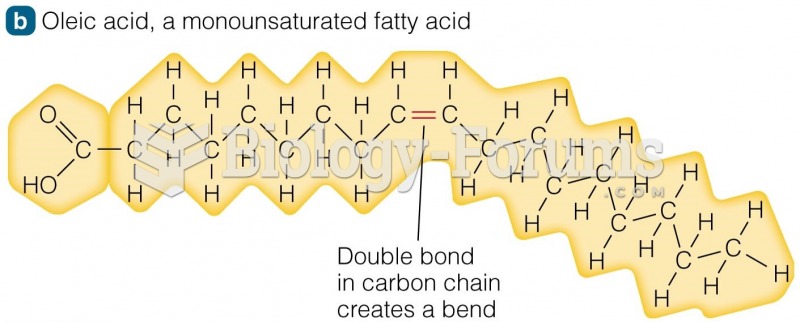
Aspirin may benefit 11 different cancers, including those of the colon, pancreas, lungs, prostate, breasts, and leukemia.
Did you know?
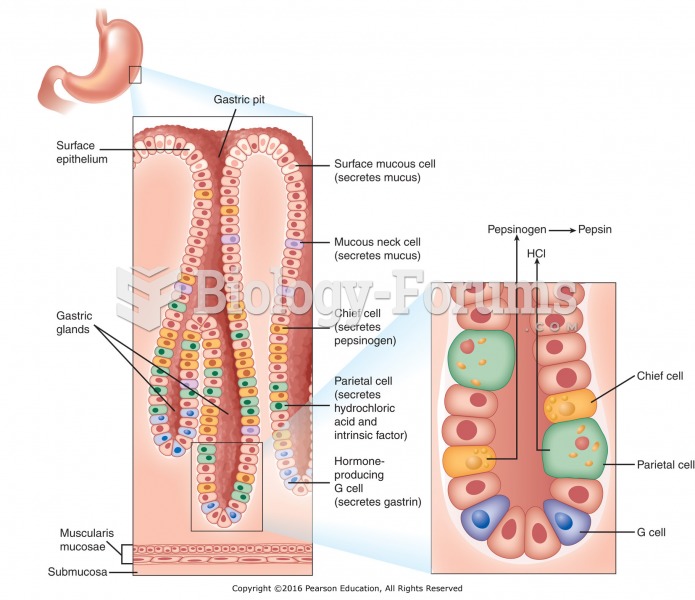
The average human gut is home to perhaps 500 to 1,000 different species of bacteria.
Did you know?
Elderly adults are living longer, and causes of death are shifting. At the same time, autopsy rates are at or near their lowest in history.