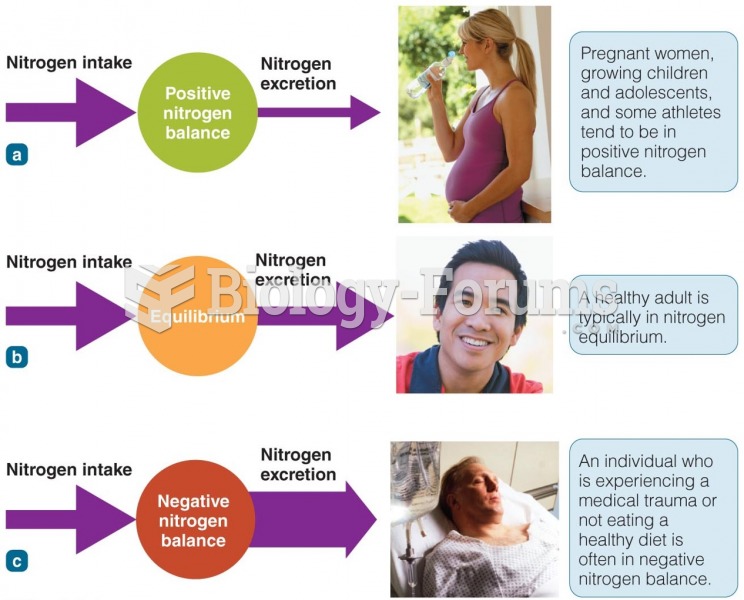
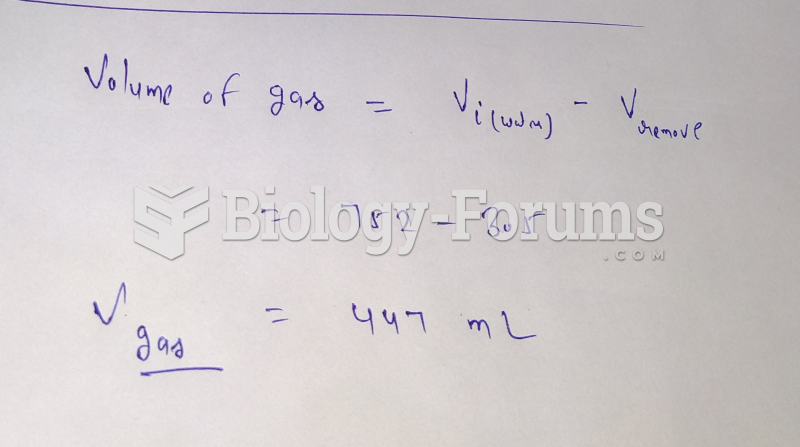
|
|
|
Your chance of developing a kidney stone is 1 in 10. In recent years, approximately 3.7 million people in the United States were diagnosed with a kidney disease.
For pediatric patients, intravenous fluids are the most commonly cited products involved in medication errors that are reported to the USP.
Urine turns bright yellow if larger than normal amounts of certain substances are consumed; one of these substances is asparagus.
The cure for trichomoniasis is easy as long as the patient does not drink alcoholic beverages for 24 hours. Just a single dose of medication is needed to rid the body of the disease. However, without proper precautions, an individual may contract the disease repeatedly. In fact, most people develop trichomoniasis again within three months of their last treatment.
Bacteria have been found alive in a lake buried one half mile under ice in Antarctica.