|
|
|
A strange skin disease referred to as Morgellons has occurred in the southern United States and in California. Symptoms include slowly healing sores, joint pain, persistent fatigue, and a sensation of things crawling through the skin. Another symptom is strange-looking, threadlike extrusions coming out of the skin.
In 2012, nearly 24 milliion Americans, aged 12 and older, had abused an illicit drug, according to the National Institute on Drug Abuse (NIDA).
The Centers for Disease Control and Prevention has released reports detailing the deaths of infants (younger than 1 year of age) who died after being given cold and cough medications. This underscores the importance of educating parents that children younger than 2 years of age should never be given over-the-counter cold and cough medications without consulting their physicians.
Barbituric acid, the base material of barbiturates, was first synthesized in 1863 by Adolph von Bayer. His company later went on to synthesize aspirin for the first time, and Bayer aspirin is still a popular brand today.
In the United States, an estimated 50 million unnecessary antibiotics are prescribed for viral respiratory infections.
 Aging is more than biology. In some cultures, Jennifer Lopez, 43, would be considered elderly. Lopez ...
Aging is more than biology. In some cultures, Jennifer Lopez, 43, would be considered elderly. Lopez ...
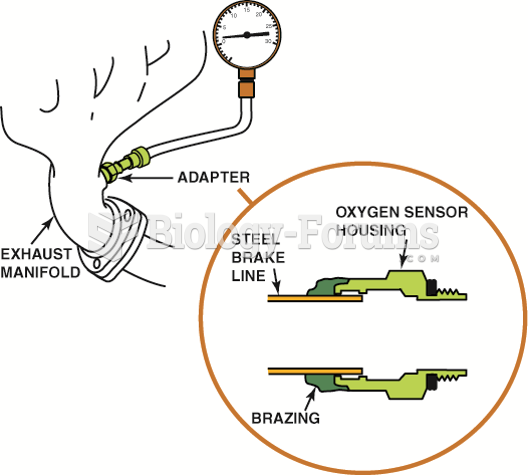
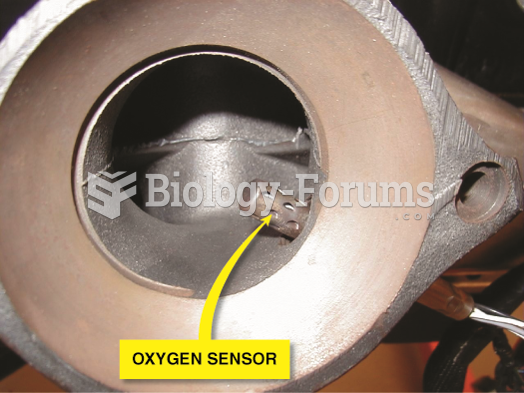 Many fuel-control oxygen sensors are located in the exhaust manifold near its outlet so that the ...
Many fuel-control oxygen sensors are located in the exhaust manifold near its outlet so that the ...