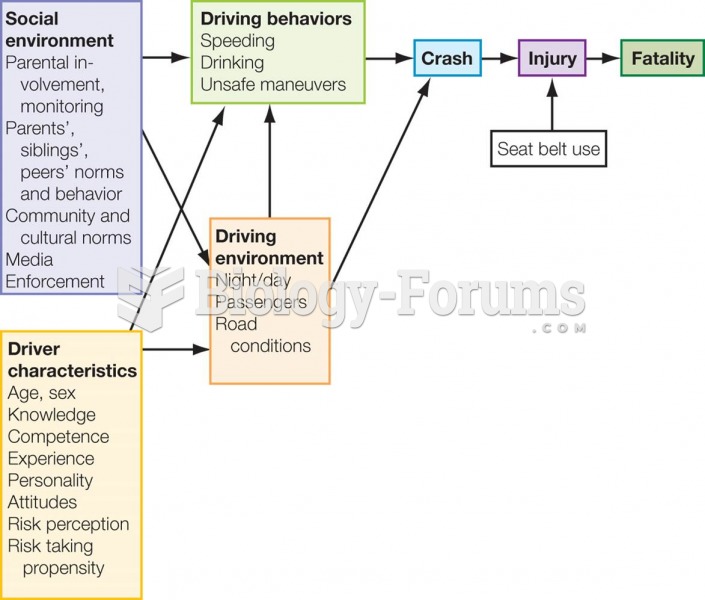
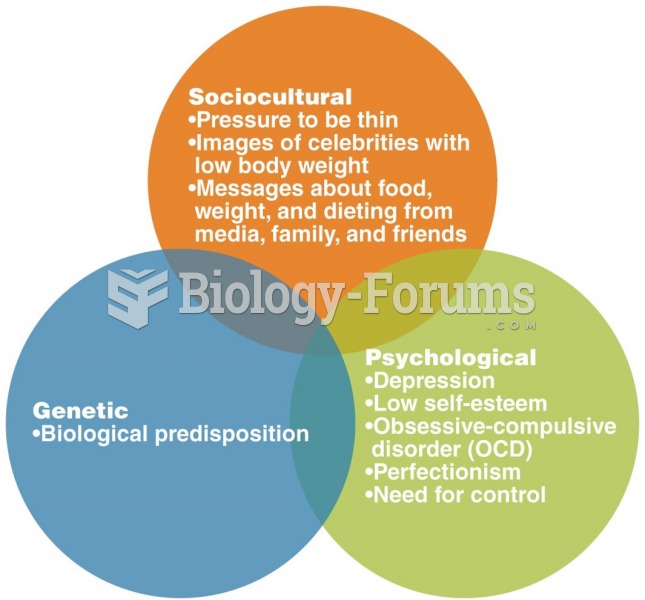
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
In ancient Rome, many of the richer people in the population had lead-induced gout. The reason for this is unclear. Lead poisoning has also been linked to madness.
Did you know?
Excessive alcohol use costs the country approximately $235 billion every year.
Did you know?
Thyroid conditions cause a higher risk of fibromyalgia and chronic fatigue syndrome.
Did you know?
The average human gut is home to perhaps 500 to 1,000 different species of bacteria.
Did you know?
The first oncogene was discovered in 1970 and was termed SRC (pronounced "SARK").