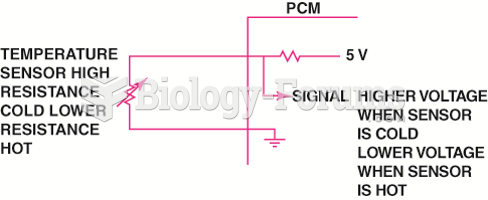
|
|
|
About 100 new prescription or over-the-counter drugs come into the U.S. market every year.
Coca-Cola originally used coca leaves and caffeine from the African kola nut. It was advertised as a therapeutic agent and "pickerupper." Eventually, its formulation was changed, and the coca leaves were removed because of the effects of regulation on cocaine-related products.
The top 10 most important tips that will help you grow old gracefully include (1) quit smoking, (2) keep your weight down, (3) take supplements, (4) skip a meal each day or fast 1 day per week, (5) get a pet, (6) get medical help for chronic pain, (7) walk regularly, (8) reduce arguments, (9) put live plants in your living space, and (10) do some weight training.
The National Institutes of Health have supported research into acupuncture. This has shown that acupuncture significantly reduced pain associated with osteoarthritis of the knee, when used as a complement to conventional therapies.
About 600,000 particles of skin are shed every hour by each human. If you live to age 70 years, you have shed 105 pounds of dead skin.
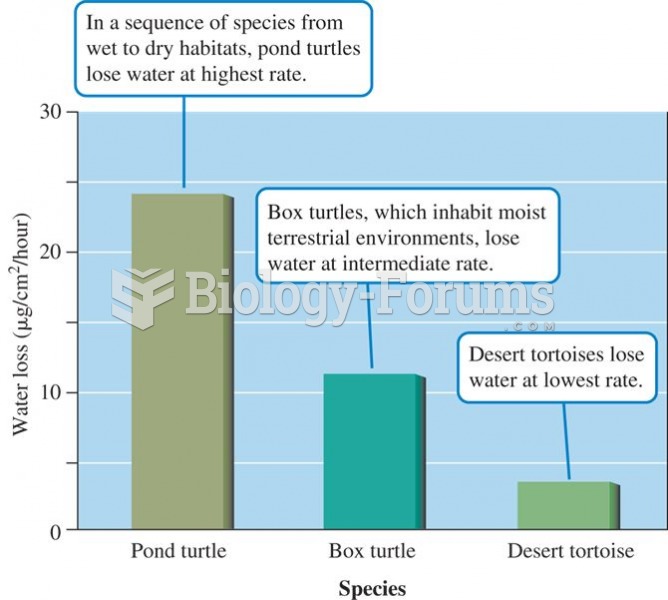 Rates of water loss by two turtles and a tortoise indicate an inverse relationship between the dryne
Rates of water loss by two turtles and a tortoise indicate an inverse relationship between the dryne
 The patient is undergoing an allergy skin test by receiving subdermal inoculations of allergens. Inf
The patient is undergoing an allergy skin test by receiving subdermal inoculations of allergens. Inf
 Becoming a Certified Medical Assistant may demonstrate your commitment to the profession and the con
Becoming a Certified Medical Assistant may demonstrate your commitment to the profession and the con