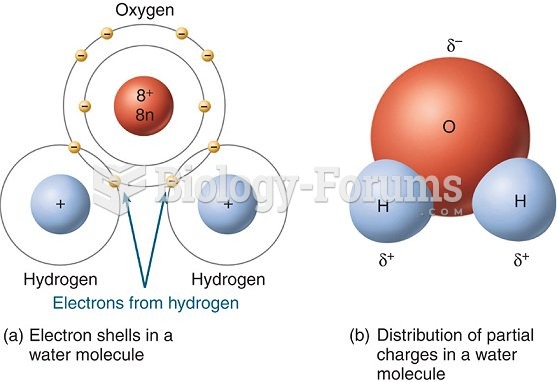
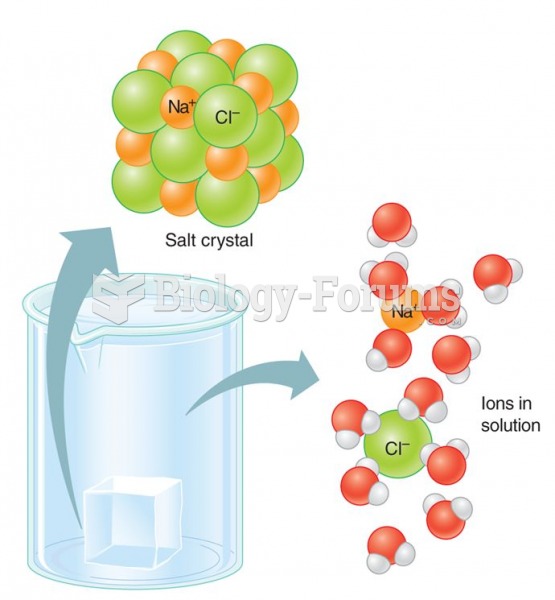
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Asthma cases in Americans are about 75% higher today than they were in 1980.
Did you know?
Urine turns bright yellow if larger than normal amounts of certain substances are consumed; one of these substances is asparagus.
Did you know?
In 2012, nearly 24 milliion Americans, aged 12 and older, had abused an illicit drug, according to the National Institute on Drug Abuse (NIDA).
Did you know?
Long-term mental and physical effects from substance abuse include: paranoia, psychosis, immune deficiencies, and organ damage.
Did you know?
Blood is approximately twice as thick as water because of the cells and other components found in it.