|
|
|
People about to have surgery must tell their health care providers about all supplements they take.
The first oral chemotherapy drug for colon cancer was approved by FDA in 2001.
More than 150,000 Americans killed by cardiovascular disease are younger than the age of 65 years.
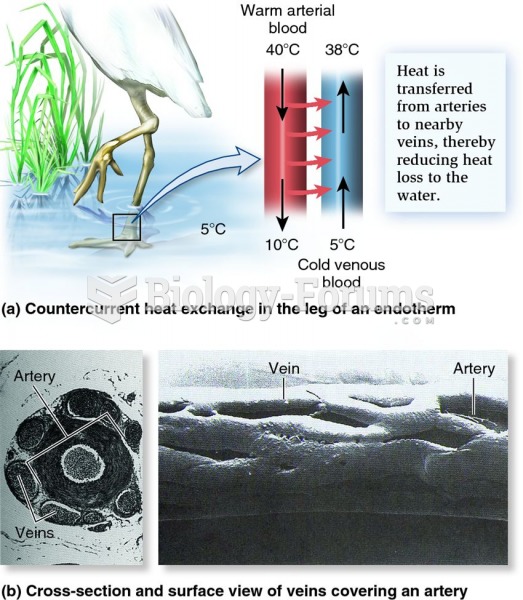
When Gabriel Fahrenheit invented the first mercury thermometer, he called "zero degrees" the lowest temperature he was able to attain with a mixture of ice and salt. For the upper point of his scale, he used 96°, which he measured as normal human body temperature (we know it to be 98.6° today because of more accurate thermometers).
In the ancient and medieval periods, dysentery killed about ? of all babies before they reach 12 months of age. The disease was transferred through contaminated drinking water, because there was no way to adequately dispose of sewage, which contaminated the water.