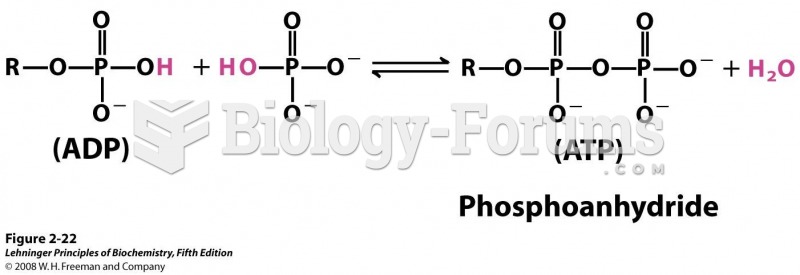
|
|
|
Thyroid conditions cause a higher risk of fibromyalgia and chronic fatigue syndrome.
Looking at the sun may not only cause headache and distort your vision temporarily, but it can also cause permanent eye damage. Any exposure to sunlight adds to the cumulative effects of ultraviolet (UV) radiation on your eyes. UV exposure has been linked to eye disorders such as macular degeneration, solar retinitis, and corneal dystrophies.
Medications that are definitely not safe to take when breastfeeding include radioactive drugs, antimetabolites, some cancer (chemotherapy) agents, bromocriptine, ergotamine, methotrexate, and cyclosporine.
The calories found in one piece of cherry cheesecake could light a 60-watt light bulb for 1.5 hours.
The average adult has about 21 square feet of skin.