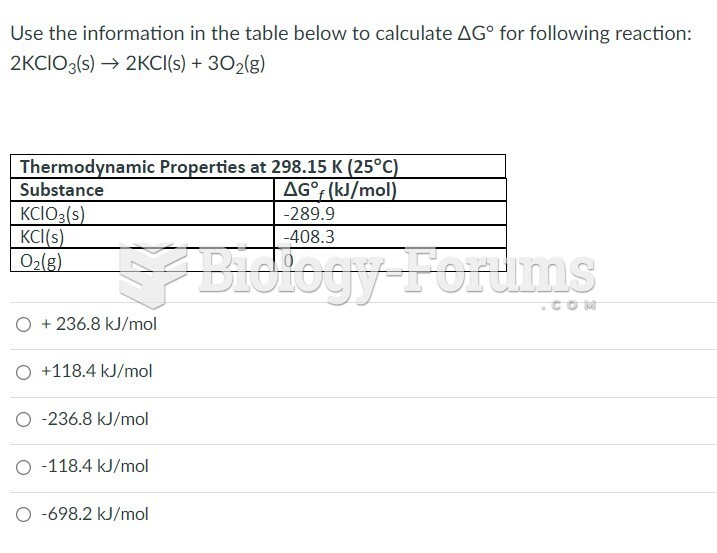
|
|
|
Increased intake of vitamin D has been shown to reduce fractures up to 25% in older people.
Your heart beats over 36 million times a year.
To prove that stomach ulcers were caused by bacteria and not by stress, a researcher consumed an entire laboratory beaker full of bacterial culture. After this, he did indeed develop stomach ulcers, and won the Nobel Prize for his discovery.
Approximately 25% of all reported medication errors result from some kind of name confusion.
A recent study has found that following a diet rich in berries may slow down the aging process of the brain. This diet apparently helps to keep dopamine levels much higher than are seen in normal individuals who do not eat berries as a regular part of their diet as they enter their later years.