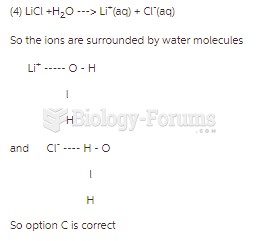
|
|
|
The Babylonians wrote numbers in a system that used 60 as the base value rather than the number 10. They did not have a symbol for "zero."
According to the FDA, adverse drug events harmed or killed approximately 1,200,000 people in the United States in the year 2015.
Common abbreviations that cause medication errors include U (unit), mg (milligram), QD (every day), SC (subcutaneous), TIW (three times per week), D/C (discharge or discontinue), HS (at bedtime or "hours of sleep"), cc (cubic centimeters), and AU (each ear).
The term bacteria was devised in the 19th century by German biologist Ferdinand Cohn. He based it on the Greek word "bakterion" meaning a small rod or staff. Cohn is considered to be the father of modern bacteriology.
Liver spots have nothing whatsoever to do with the liver. They are a type of freckles commonly seen in older adults who have been out in the sun without sufficient sunscreen.