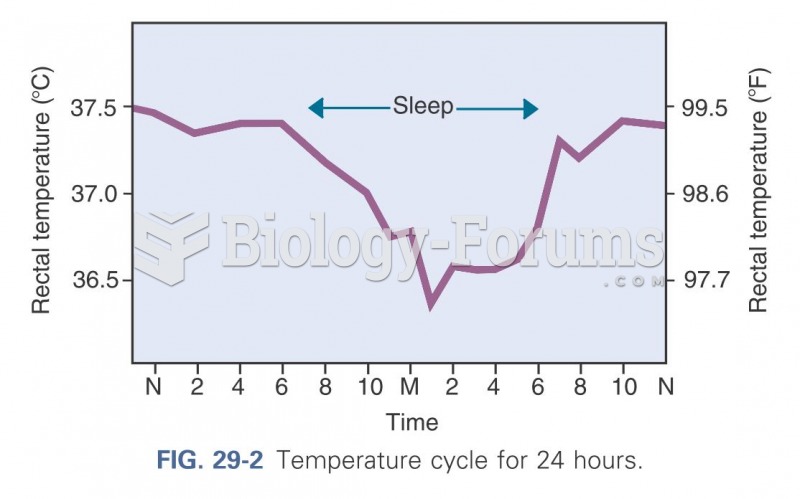
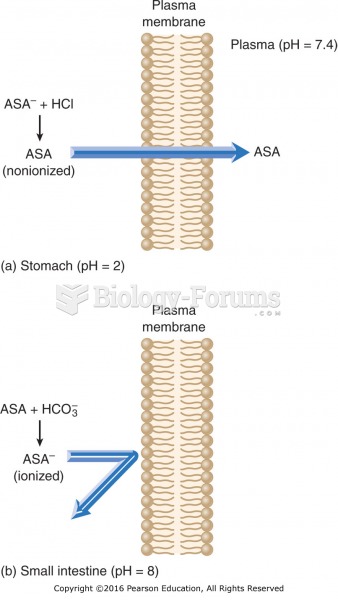
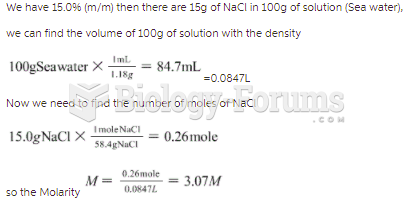|
|
|
The average person is easily confused by the terms pharmaceutics and pharmacology, thinking they are one and the same. Whereas pharmaceutics is the science of preparing and dispensing drugs (otherwise known as the science of pharmacy), pharmacology is the study of medications.
Increased intake of vitamin D has been shown to reduce fractures up to 25% in older people.
The first documented use of surgical anesthesia in the United States was in Connecticut in 1844.
Illicit drug use costs the United States approximately $181 billion every year.
Vaccines cause herd immunity. If the majority of people in a community have been vaccinated against a disease, an unvaccinated person is less likely to get the disease since others are less likely to become sick from it and spread the disease.