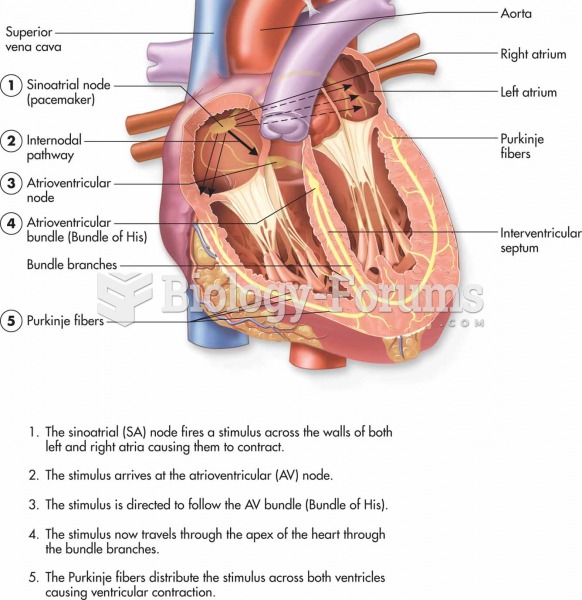
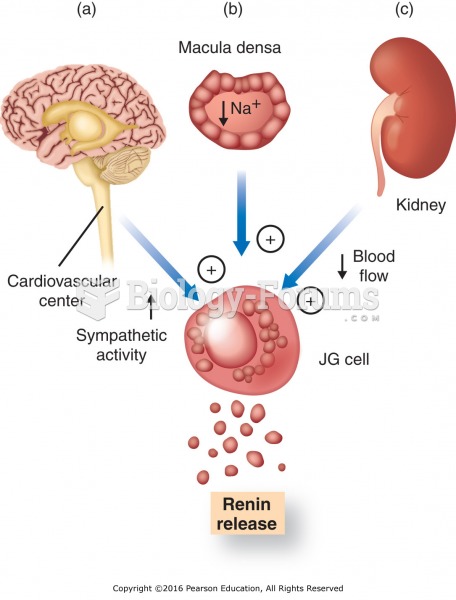
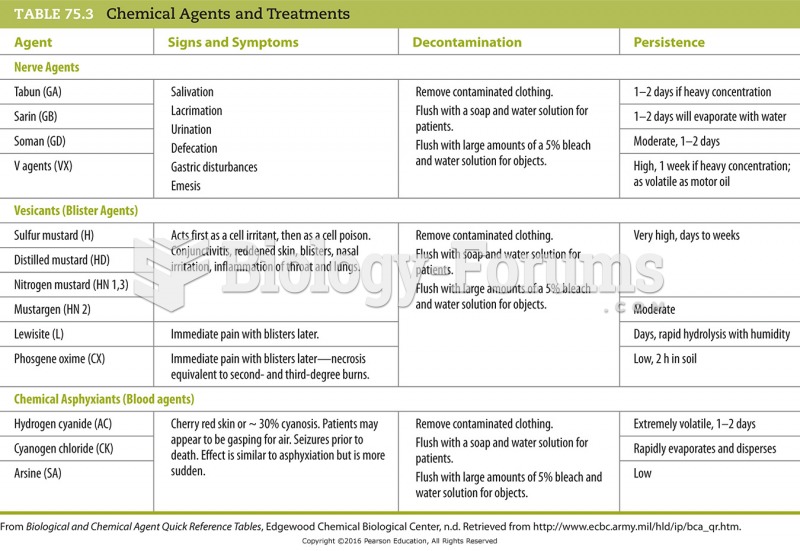
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Sperm cells are so tiny that 400 to 500 million (400,000,000–500,000,000) of them fit onto 1 tsp.
Did you know?
The average office desk has 400 times more bacteria on it than a toilet.
Did you know?
Long-term mental and physical effects from substance abuse include: paranoia, psychosis, immune deficiencies, and organ damage.
Did you know?
One way to reduce acid reflux is to lose two or three pounds. Most people lose weight in the belly area first when they increase exercise, meaning that heartburn can be reduced quickly by this method.
Did you know?
Limit intake of red meat and dairy products made with whole milk. Choose skim milk, low-fat or fat-free dairy products. Limit fried food. Use healthy oils when cooking.