|
|
|
About 3.2 billion people, nearly half the world population, are at risk for malaria. In 2015, there are about 214 million malaria cases and an estimated 438,000 malaria deaths.
There are 60,000 miles of blood vessels in every adult human.
Urine turns bright yellow if larger than normal amounts of certain substances are consumed; one of these substances is asparagus.
The Centers for Disease Control and Prevention (CDC) was originally known as the Communicable Disease Center, which was formed to fight malaria. It was originally headquartered in Atlanta, Georgia, since the Southern states faced the worst threat from malaria.
Parkinson's disease is both chronic and progressive. This means that it persists over a long period of time and that its symptoms grow worse over time.
 Critical thinking involves analysis in which the nurse examines patient data available from a variet
Critical thinking involves analysis in which the nurse examines patient data available from a variet
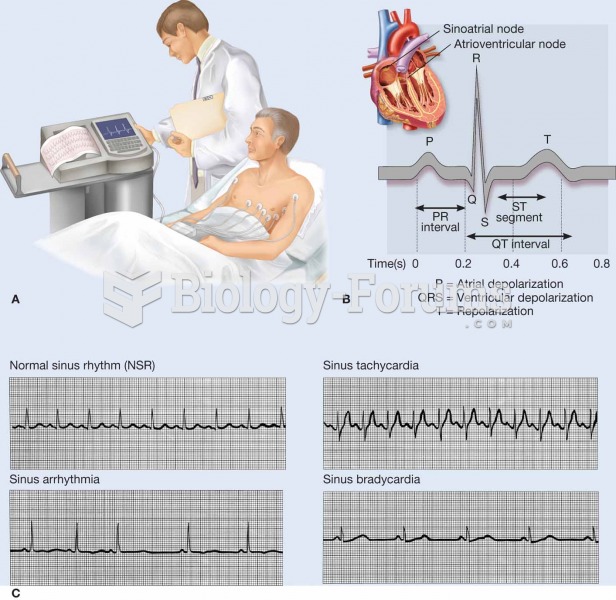 An electrocardiogram (ECG, EKG) is a commonly used procedure in which the electrical events associat
An electrocardiogram (ECG, EKG) is a commonly used procedure in which the electrical events associat
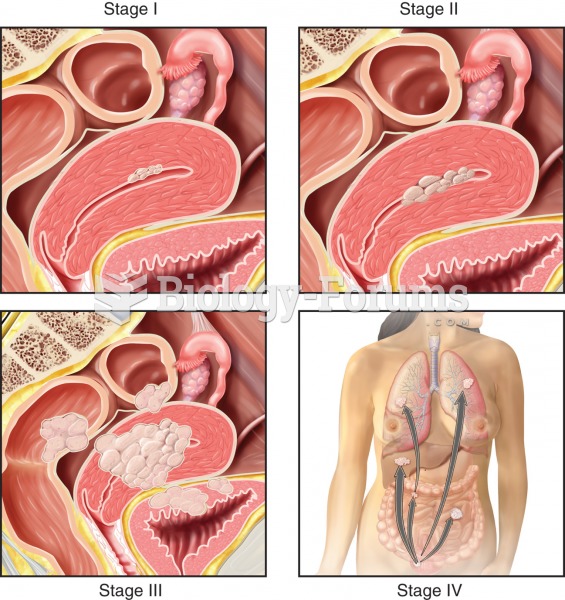 Stages of endometrial cancer. Stage I: Mutated cells arise from glandular epithelium of the endometr
Stages of endometrial cancer. Stage I: Mutated cells arise from glandular epithelium of the endometr