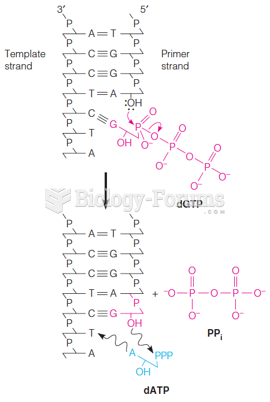
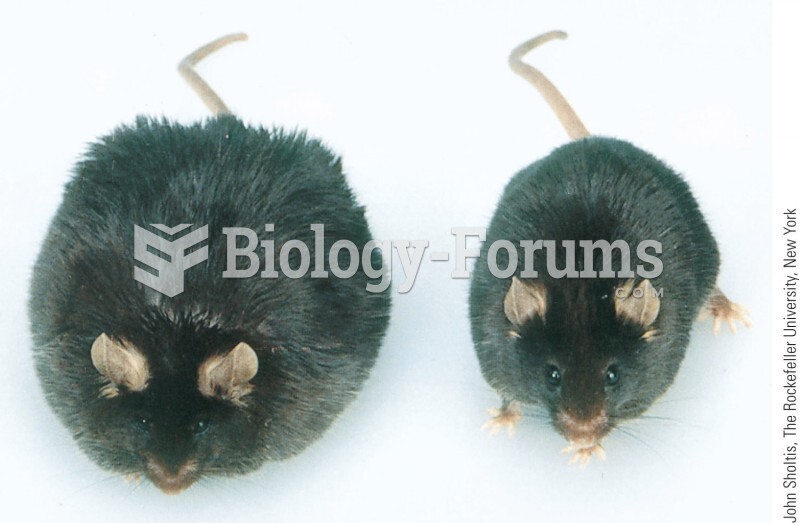
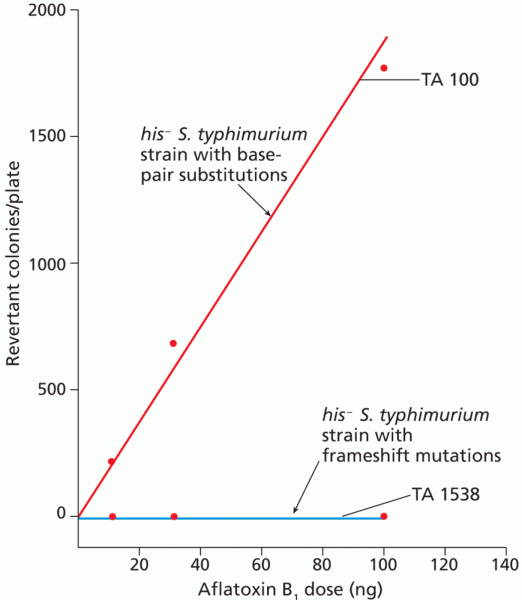
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Most fungi that pathogenically affect humans live in soil. If a person is not healthy, has an open wound, or is immunocompromised, a fungal infection can be very aggressive.
Did you know?
Aspirin is the most widely used drug in the world. It has even been recognized as such by the Guinness Book of World Records.
Did you know?
Urine turns bright yellow if larger than normal amounts of certain substances are consumed; one of these substances is asparagus.
Did you know?
To combat osteoporosis, changes in lifestyle and diet are recommended. At-risk patients should include 1,200 to 1,500 mg of calcium daily either via dietary means or with supplements.
Did you know?
Illicit drug use costs the United States approximately $181 billion every year.
 Native American casinos remain a topic of both controversy and envy. Shown here is Corey Two Crow as ...
Native American casinos remain a topic of both controversy and envy. Shown here is Corey Two Crow as ...
 The material safety data sheet (MSDS) for sulfuric acid showing the detailed technical information ...
The material safety data sheet (MSDS) for sulfuric acid showing the detailed technical information ...