|
|
|
Asthma-like symptoms were first recorded about 3,500 years ago in Egypt. The first manuscript specifically written about asthma was in the year 1190, describing a condition characterized by sudden breathlessness. The treatments listed in this manuscript include chicken soup, herbs, and sexual abstinence.
The cure for trichomoniasis is easy as long as the patient does not drink alcoholic beverages for 24 hours. Just a single dose of medication is needed to rid the body of the disease. However, without proper precautions, an individual may contract the disease repeatedly. In fact, most people develop trichomoniasis again within three months of their last treatment.
The tallest man ever known was Robert Wadlow, an American, who reached the height of 8 feet 11 inches. He died at age 26 years from an infection caused by the immense weight of his body (491 pounds) and the stress on his leg bones and muscles.
The calories found in one piece of cherry cheesecake could light a 60-watt light bulb for 1.5 hours.
Russia has the highest death rate from cardiovascular disease followed by the Ukraine, Romania, Hungary, and Poland.
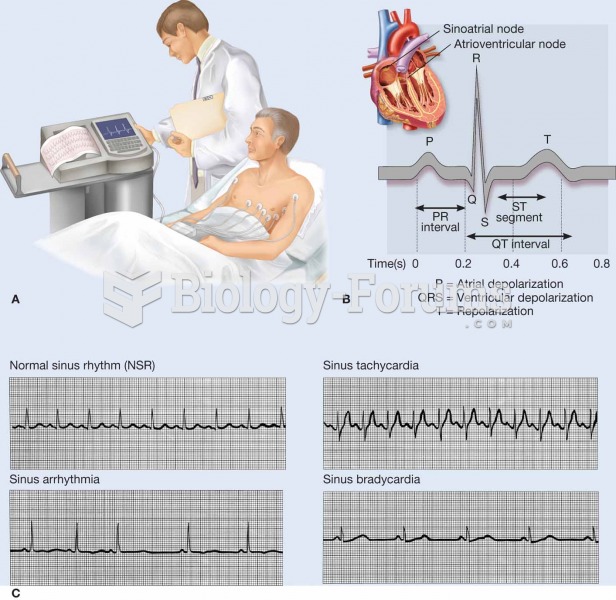 An electrocardiogram (ECG, EKG) is a commonly used procedure in which the electrical events associat
An electrocardiogram (ECG, EKG) is a commonly used procedure in which the electrical events associat
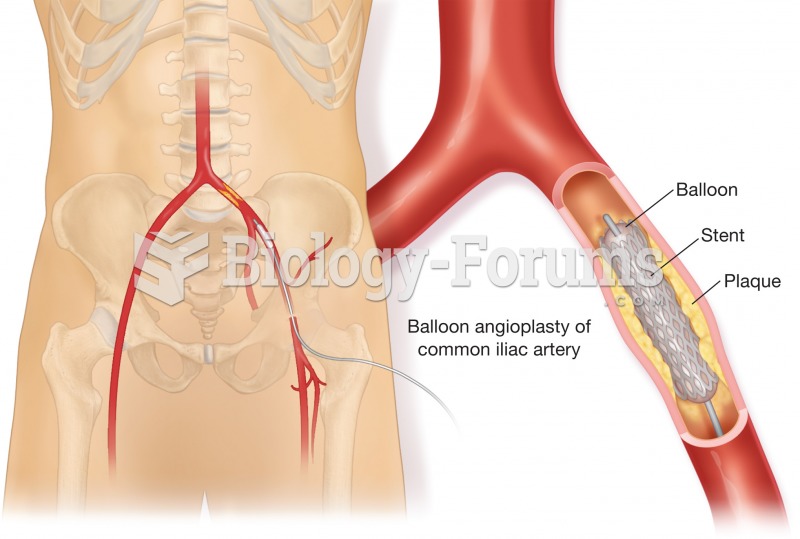 Angioplasty. One form is called balloon angioplasty, shown here. A balloon catheter is threaded into
Angioplasty. One form is called balloon angioplasty, shown here. A balloon catheter is threaded into