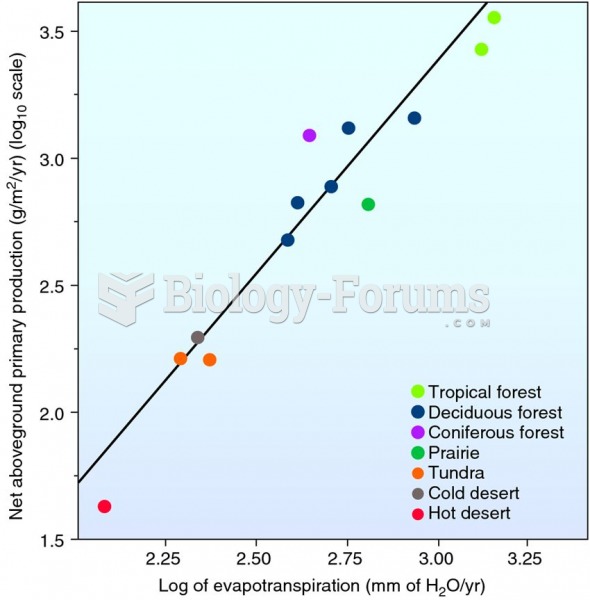
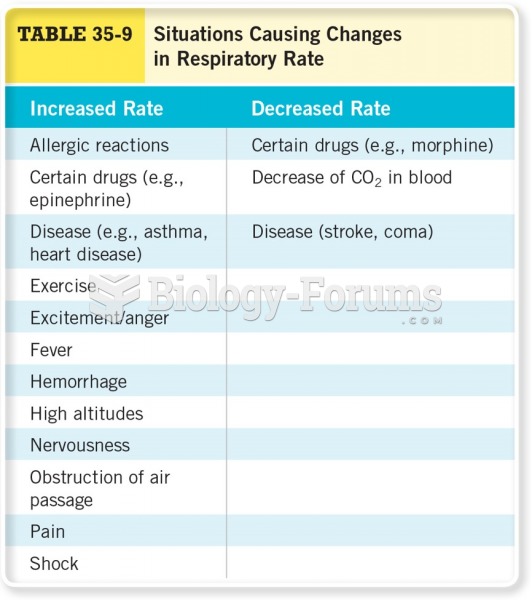
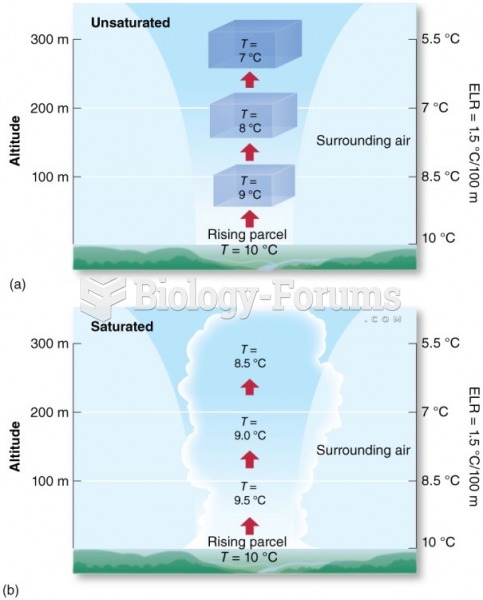
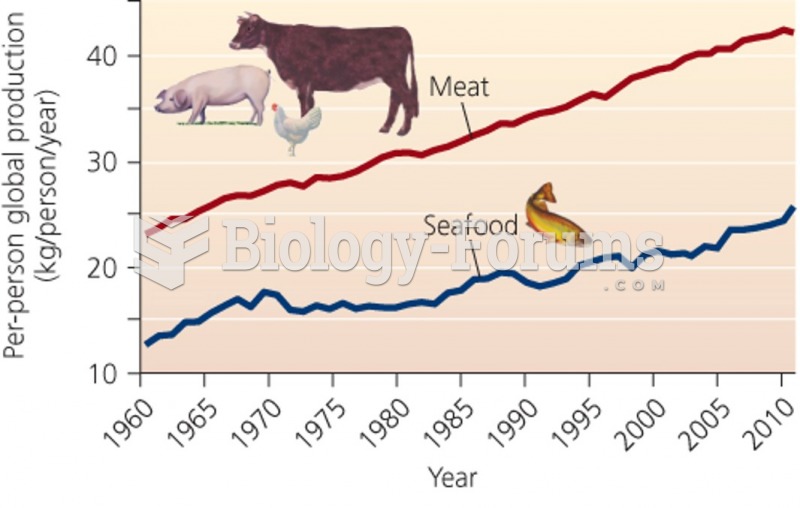
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Today, nearly 8 out of 10 pregnant women living with HIV (about 1.1 million), receive antiretrovirals.
Did you know?
The familiar sounds of your heart are made by the heart's valves as they open and close.
Did you know?
In the United States, there is a birth every 8 seconds, according to the U.S. Census Bureau's Population Clock.
Did you know?
Earwax has antimicrobial properties that reduce the viability of bacteria and fungus in the human ear.
Did you know?
All adverse reactions are commonly charted in red ink in the patient's record and usually are noted on the front of the chart. Failure to follow correct documentation procedures may result in malpractice lawsuits.