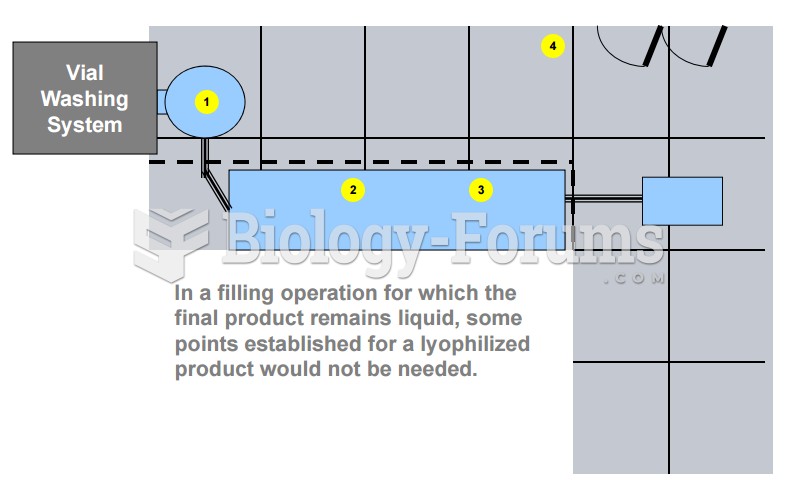
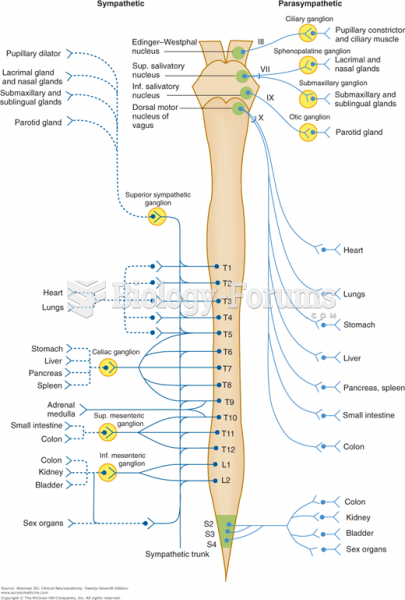
|
|
|
Malaria mortality rates are falling. Increased malaria prevention and control measures have greatly improved these rates. Since 2000, malaria mortality rates have fallen globally by 60% among all age groups, and by 65% among children under age 5.
In 1864, the first barbiturate (barbituric acid) was synthesized.
If you could remove all of your skin, it would weigh up to 5 pounds.
Asthma-like symptoms were first recorded about 3,500 years ago in Egypt. The first manuscript specifically written about asthma was in the year 1190, describing a condition characterized by sudden breathlessness. The treatments listed in this manuscript include chicken soup, herbs, and sexual abstinence.
Normal urine is sterile. It contains fluids, salts, and waste products. It is free of bacteria, viruses, and fungi.
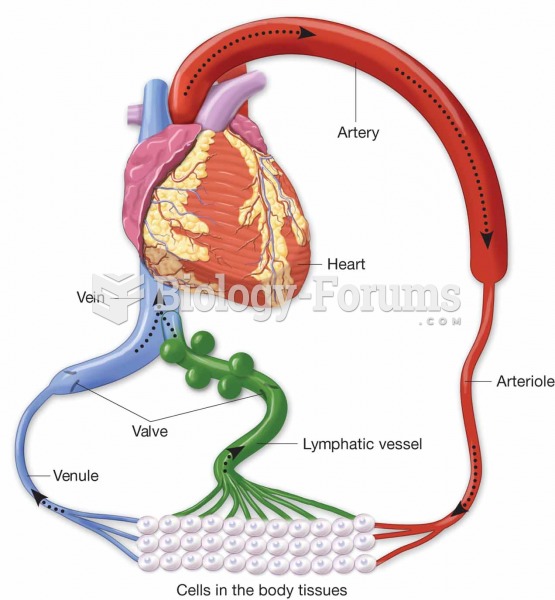 Lymphatic vessels pick up excess tissue fluid, purify it in the lymph nodes, and then return it to t
Lymphatic vessels pick up excess tissue fluid, purify it in the lymph nodes, and then return it to t
 Pressure testing the cooling system. A typical hand-operated pressure tester applies pressure equal ...
Pressure testing the cooling system. A typical hand-operated pressure tester applies pressure equal ...