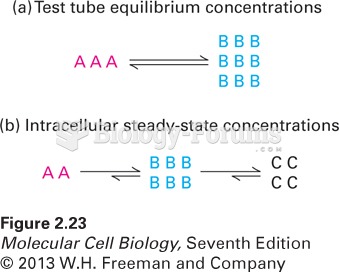
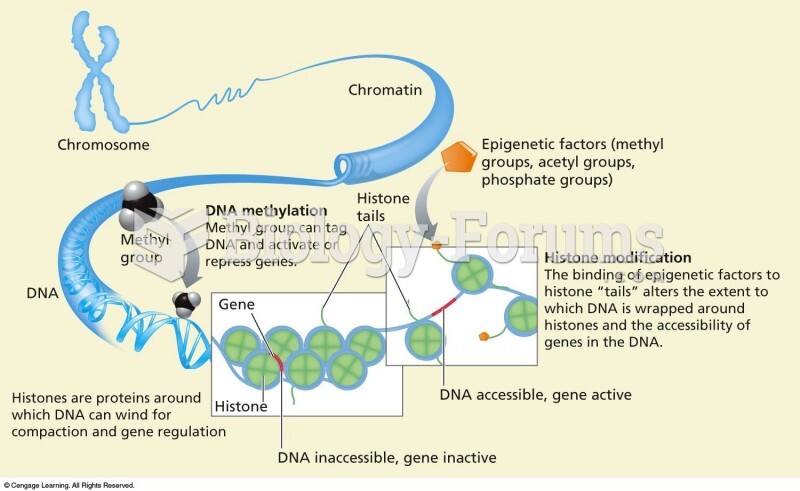
|
|
|
Asthma cases in Americans are about 75% higher today than they were in 1980.
The FDA recognizes 118 routes of administration.
Although the Roman numeral for the number 4 has always been taught to have been "IV," according to historians, the ancient Romans probably used "IIII" most of the time. This is partially backed up by the fact that early grandfather clocks displayed IIII for the number 4 instead of IV. Early clockmakers apparently thought that the IIII balanced out the VIII (used for the number 8) on the clock face and that it just looked better.
Patients who have been on total parenteral nutrition for more than a few days may need to have foods gradually reintroduced to give the digestive tract time to start working again.
Increased intake of vitamin D has been shown to reduce fractures up to 25% in older people.