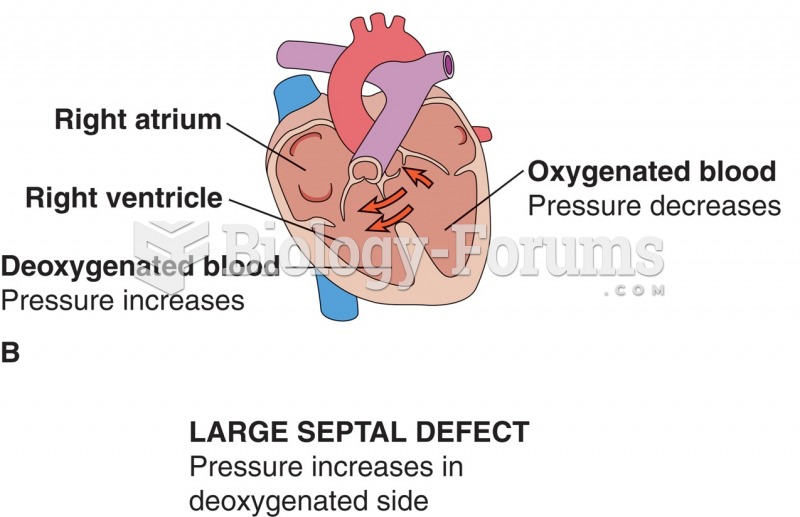
|
|
|
Asthma cases in Americans are about 75% higher today than they were in 1980.
According to the CDC, approximately 31.7% of the U.S. population has high low-density lipoprotein (LDL) or "bad cholesterol" levels.
As the western states of America were settled, pioneers often had to drink rancid water from ponds and other sources. This often resulted in chronic diarrhea, causing many cases of dehydration and death that could have been avoided if clean water had been available.
The oldest recorded age was 122. Madame Jeanne Calment was born in France in 1875 and died in 1997. She was a vegetarian and loved olive oil, port wine, and chocolate.
The cure for trichomoniasis is easy as long as the patient does not drink alcoholic beverages for 24 hours. Just a single dose of medication is needed to rid the body of the disease. However, without proper precautions, an individual may contract the disease repeatedly. In fact, most people develop trichomoniasis again within three months of their last treatment.
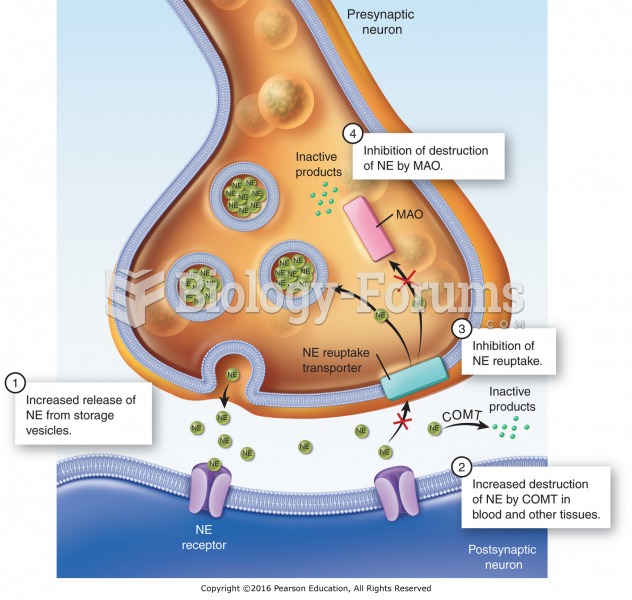 Mechanisms of action of adrenergic agonists: (1) stimulation of the release of NE; (2) increased ...
Mechanisms of action of adrenergic agonists: (1) stimulation of the release of NE; (2) increased ...
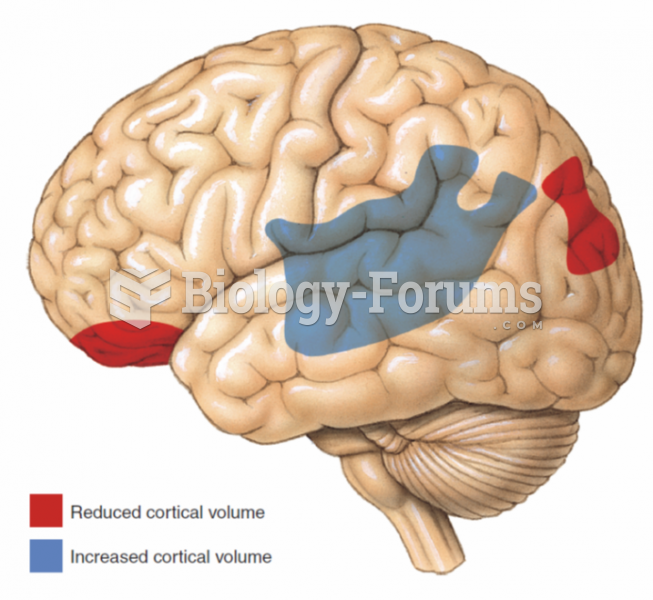 Two areas of reduced cortical volume and one area of increased cortical volume observed in people ...
Two areas of reduced cortical volume and one area of increased cortical volume observed in people ...