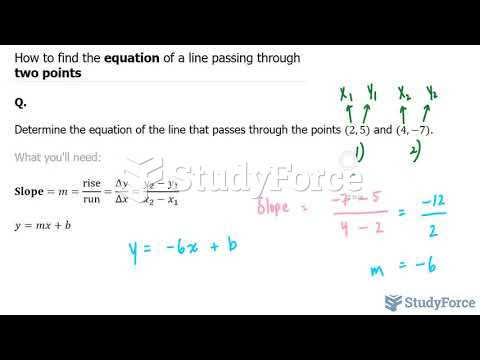
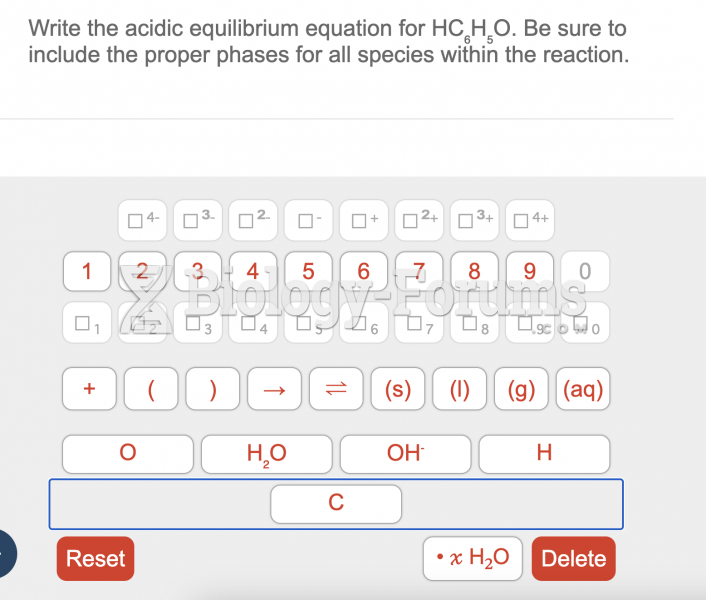
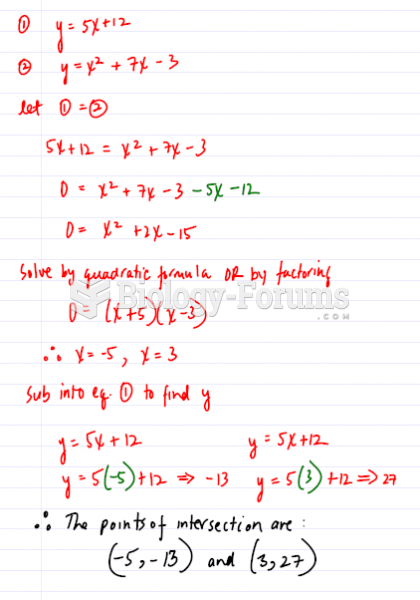This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
About 100 new prescription or over-the-counter drugs come into the U.S. market every year.
Did you know?
The senior population grows every year. Seniors older than 65 years of age now comprise more than 13% of the total population. However, women outlive men. In the 85-and-over age group, there are only 45 men to every 100 women.
Did you know?
Colchicine is a highly poisonous alkaloid originally extracted from a type of saffron plant that is used mainly to treat gout.
Did you know?
This year, an estimated 1.4 million Americans will have a new or recurrent heart attack.
Did you know?
Autoimmune diseases occur when the immune system destroys its own healthy tissues. When this occurs, white blood cells cannot distinguish between pathogens and normal cells.