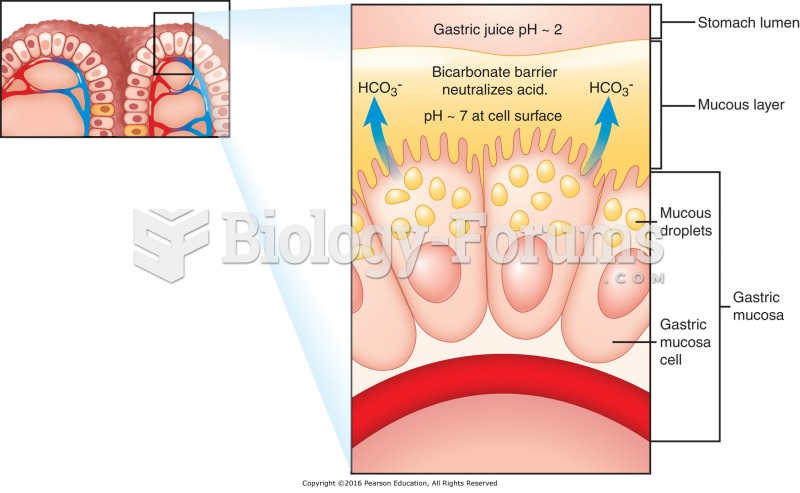
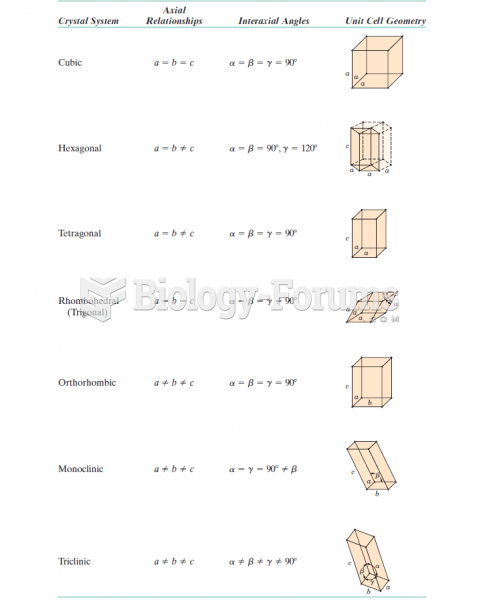
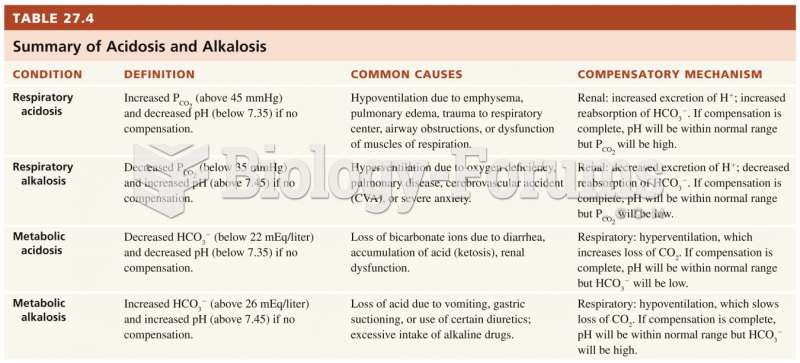
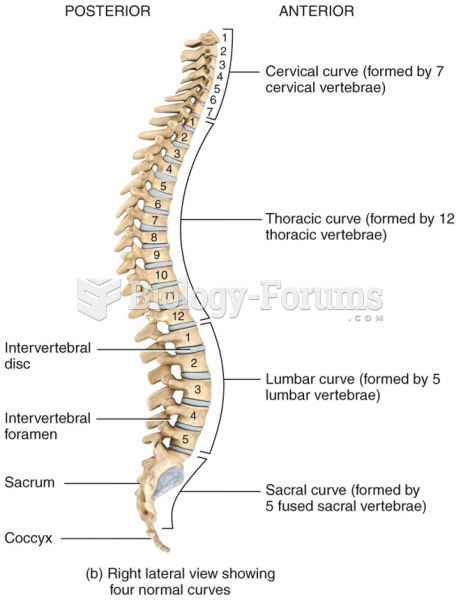
|
|
|
An identified risk factor for osteoporosis is the intake of excessive amounts of vitamin A. Dietary intake of approximately double the recommended daily amount of vitamin A, by women, has been shown to reduce bone mineral density and increase the chances for hip fractures compared with women who consumed the recommended daily amount (or less) of vitamin A.
On average, someone in the United States has a stroke about every 40 seconds. This is about 795,000 people per year.
More than nineteen million Americans carry the factor V gene that causes blood clots, pulmonary embolism, and heart disease.
Long-term mental and physical effects from substance abuse include: paranoia, psychosis, immune deficiencies, and organ damage.
Earwax has antimicrobial properties that reduce the viability of bacteria and fungus in the human ear.