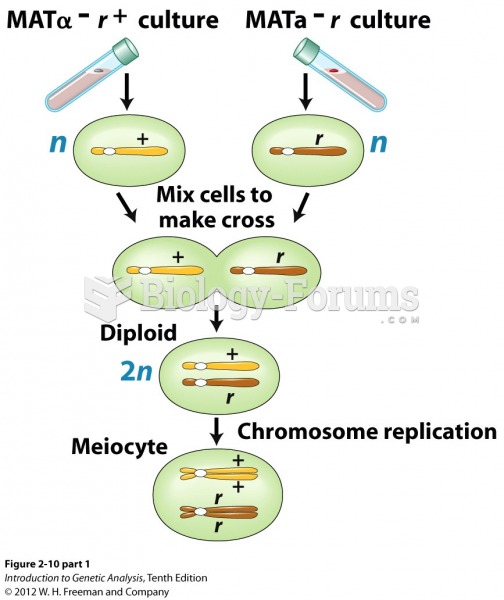
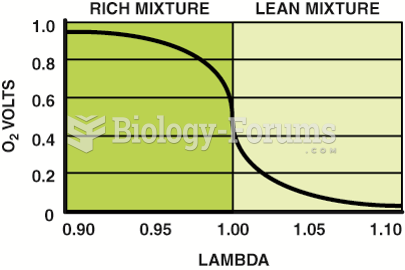
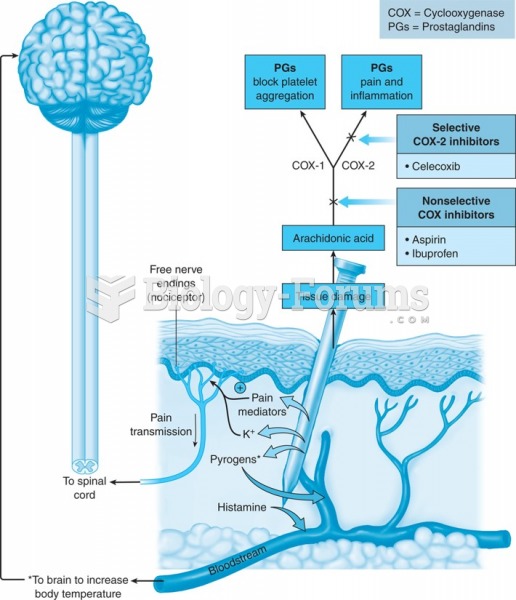
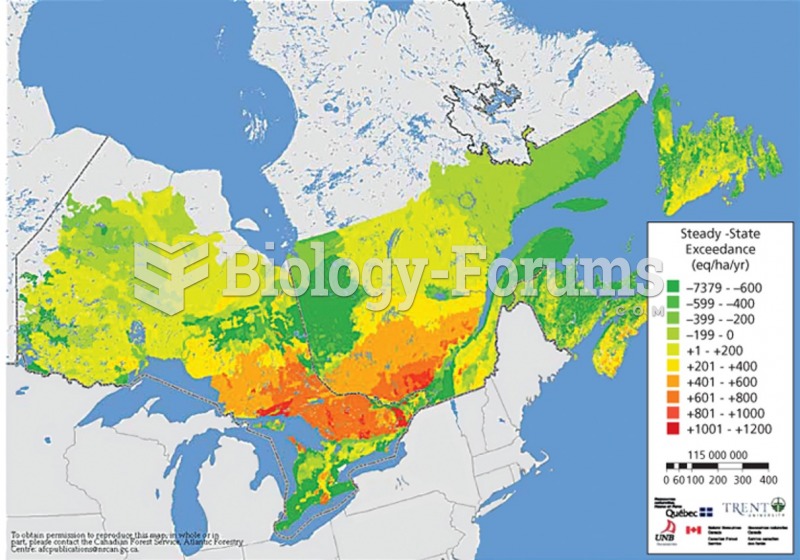
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
People with high total cholesterol have about two times the risk for heart disease as people with ideal levels.
Did you know?
Eat fiber! A diet high in fiber can help lower cholesterol levels by as much as 10%.
Did you know?
Alzheimer's disease affects only about 10% of people older than 65 years of age. Most forms of decreased mental function and dementia are caused by disuse (letting the mind get lazy).
Did you know?
Patients should never assume they are being given the appropriate drugs. They should make sure they know which drugs are being prescribed, and always double-check that the drugs received match the prescription.
Did you know?
The calories found in one piece of cherry cheesecake could light a 60-watt light bulb for 1.5 hours.