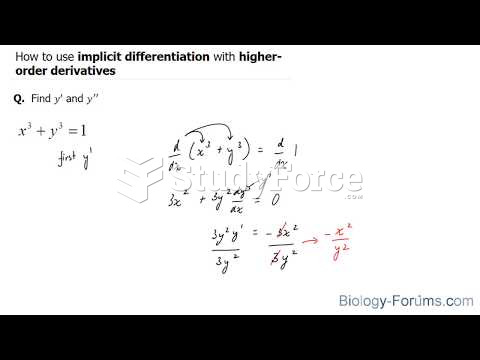
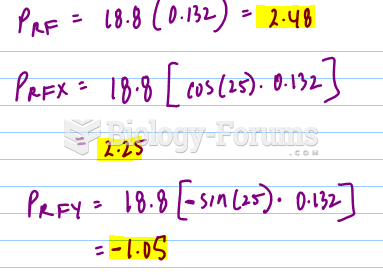This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Urine turns bright yellow if larger than normal amounts of certain substances are consumed; one of these substances is asparagus.
Did you know?
Signs and symptoms of a drug overdose include losing consciousness, fever or sweating, breathing problems, abnormal pulse, and changes in skin color.
Did you know?
After 5 years of being diagnosed with rheumatoid arthritis, one every three patients will no longer be able to work.
Did you know?
The B-complex vitamins and vitamin C are not stored in the body and must be replaced each day.
Did you know?
During the twentieth century, a variant of the metric system was used in Russia and France in which the base unit of mass was the tonne. Instead of kilograms, this system used millitonnes (mt).