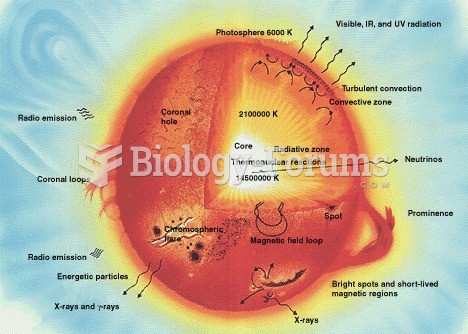
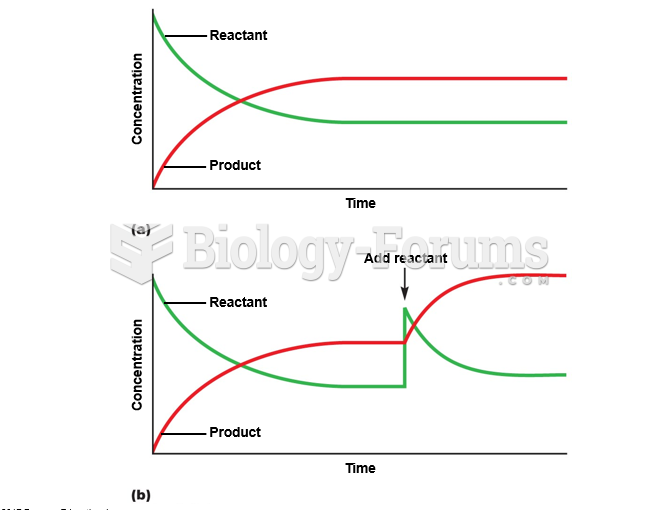
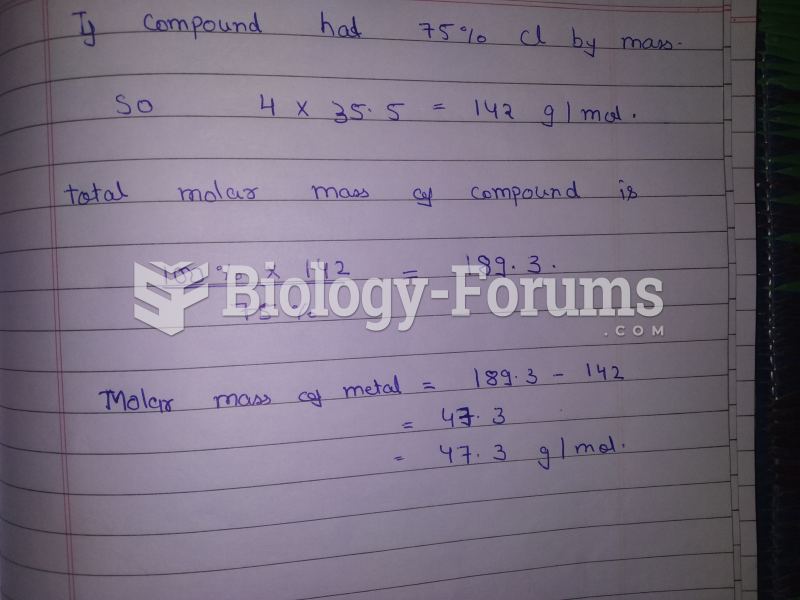
|
|
|
There can actually be a 25-hour time difference between certain locations in the world. The International Date Line passes between the islands of Samoa and American Samoa. It is not a straight line, but "zig-zags" around various island chains. Therefore, Samoa and nearby islands have one date, while American Samoa and nearby islands are one day behind. Daylight saving time is used in some islands, but not in others—further shifting the hours out of sync with natural time.
Human stomach acid is strong enough to dissolve small pieces of metal such as razor blades or staples.
The first successful kidney transplant was performed in 1954 and occurred in Boston. A kidney from an identical twin was transplanted into his dying brother's body and was not rejected because it did not appear foreign to his body.
Adults are resistant to the bacterium that causes Botulism. These bacteria thrive in honey – therefore, honey should never be given to infants since their immune systems are not yet resistant.
Asthma attacks and symptoms usually get started by specific triggers (such as viruses, allergies, gases, and air particles). You should talk to your doctor about these triggers and find ways to avoid or get rid of them.