This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The longest a person has survived after a heart transplant is 24 years.
Did you know?
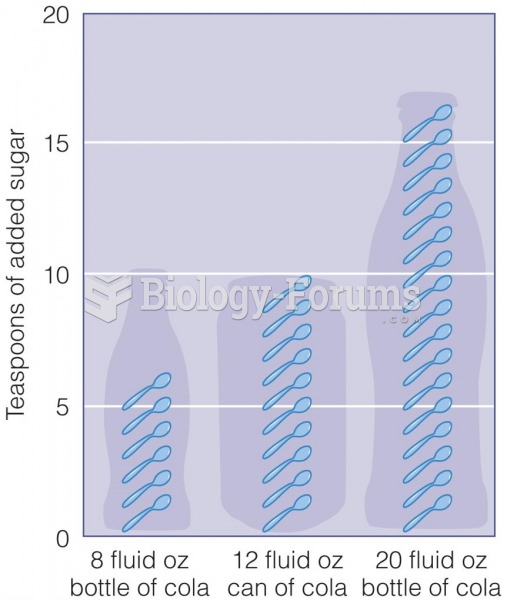
It is important to read food labels and choose foods with low cholesterol and saturated trans fat. You should limit saturated fat to no higher than 6% of daily calories.
Did you know?
Malaria was not eliminated in the United States until 1951. The term eliminated means that no new cases arise in a country for 3 years.
Did you know?
If you could remove all of your skin, it would weigh up to 5 pounds.
Did you know?
The average older adult in the United States takes five prescription drugs per day. Half of these drugs contain a sedative. Alcohol should therefore be avoided by most senior citizens because of the dangerous interactions between alcohol and sedatives.