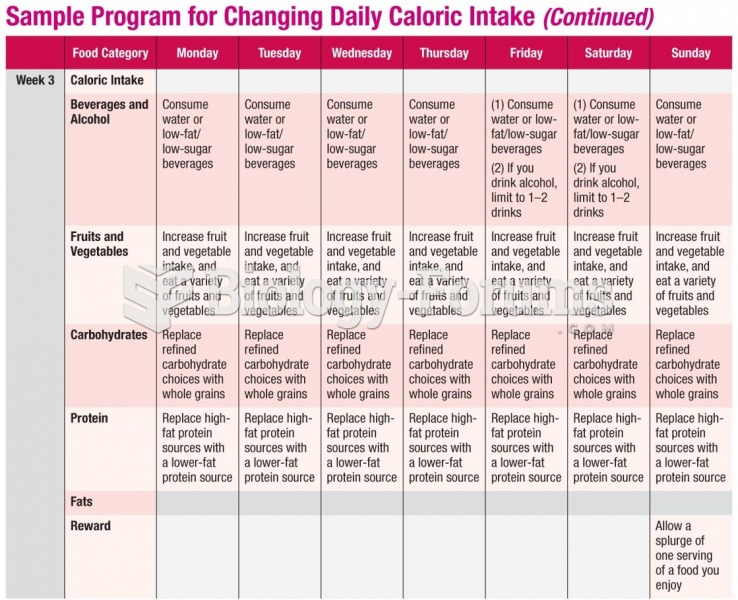
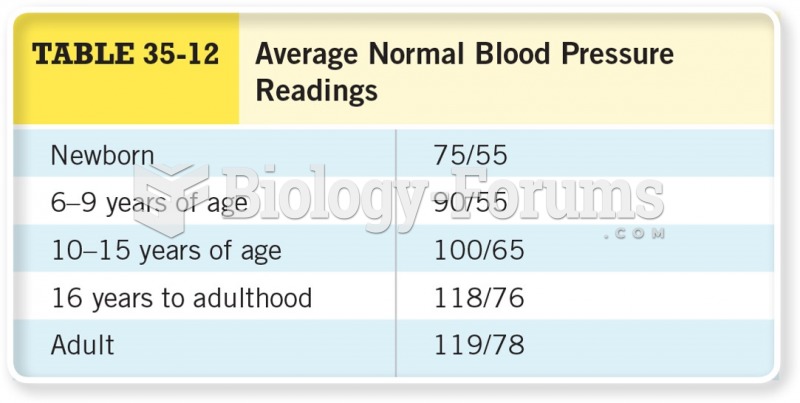
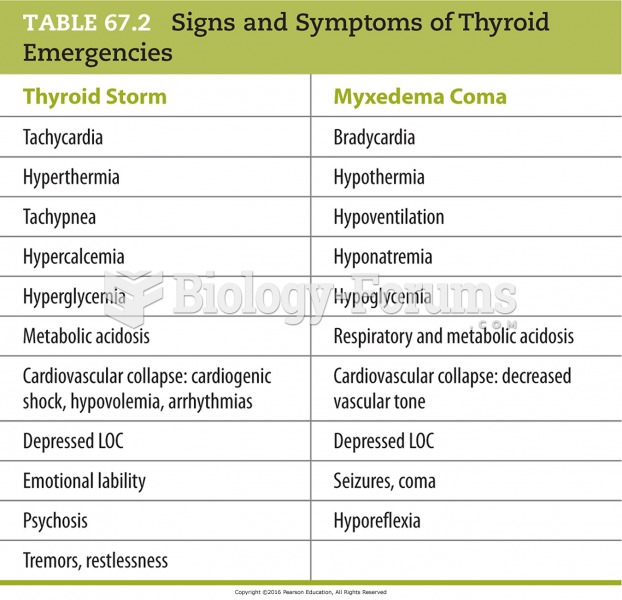
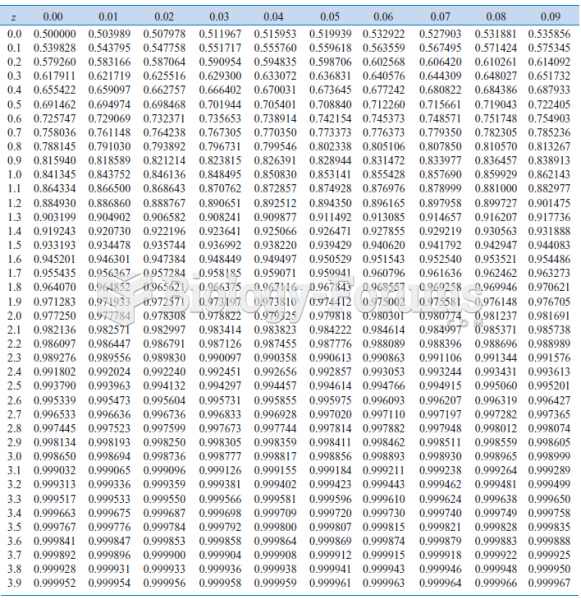
|
|
|
Approximately one in four people diagnosed with diabetes will develop foot problems. Of these, about one-third will require lower extremity amputation.
For pediatric patients, intravenous fluids are the most commonly cited products involved in medication errors that are reported to the USP.
Patients who have undergone chemotherapy for the treatment of cancer often complain of a lack of mental focus; memory loss; and a general diminution in abilities such as multitasking, attention span, and general mental agility.
More than 30% of American adults, and about 12% of children utilize health care approaches that were developed outside of conventional medicine.
Human stomach acid is strong enough to dissolve small pieces of metal such as razor blades or staples.