This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
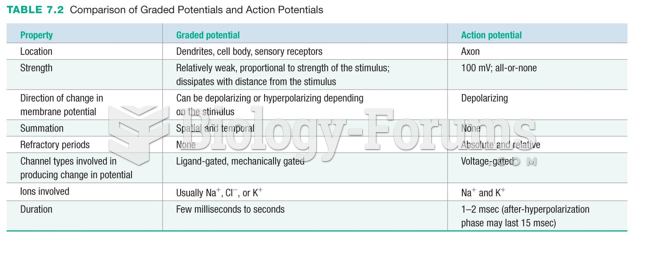
The lipid bilayer is made of phospholipids. They are arranged in a double layer because one of their ends is attracted to water while the other is repelled by water.
Did you know?
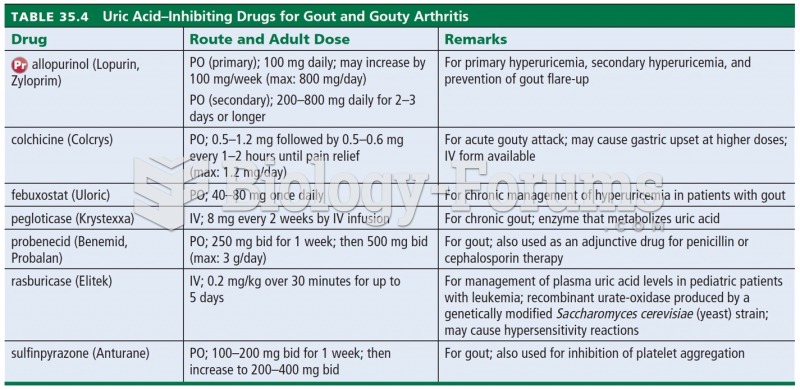
Many of the drugs used by neuroscientists are derived from toxic plants and venomous animals (such as snakes, spiders, snails, and puffer fish).
Did you know?
The heart is located in the center of the chest, with part of it tipped slightly so that it taps against the left side of the chest.
Did you know?
Carbamazepine can interfere with the results of home pregnancy tests. If you are taking carbamazepine, do not try to test for pregnancy at home.
Did you know?
Nearly 31 million adults in America have a total cholesterol level that is more than 240 mg per dL.