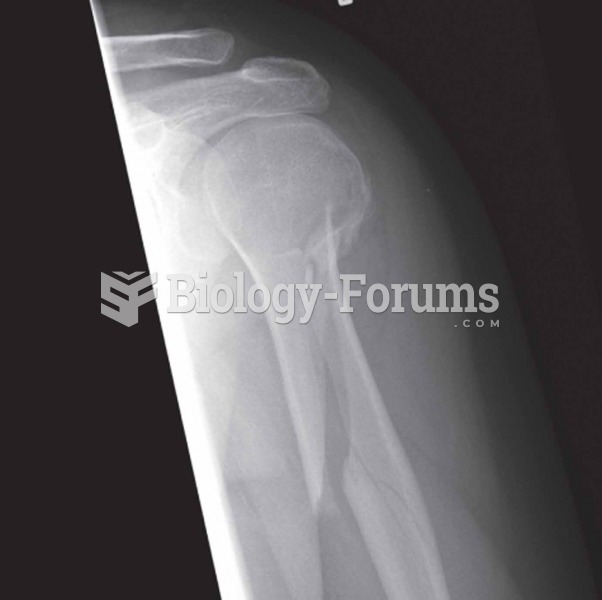
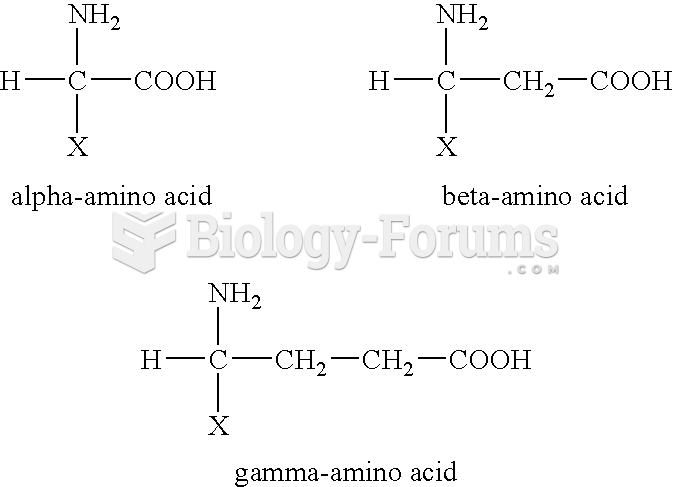
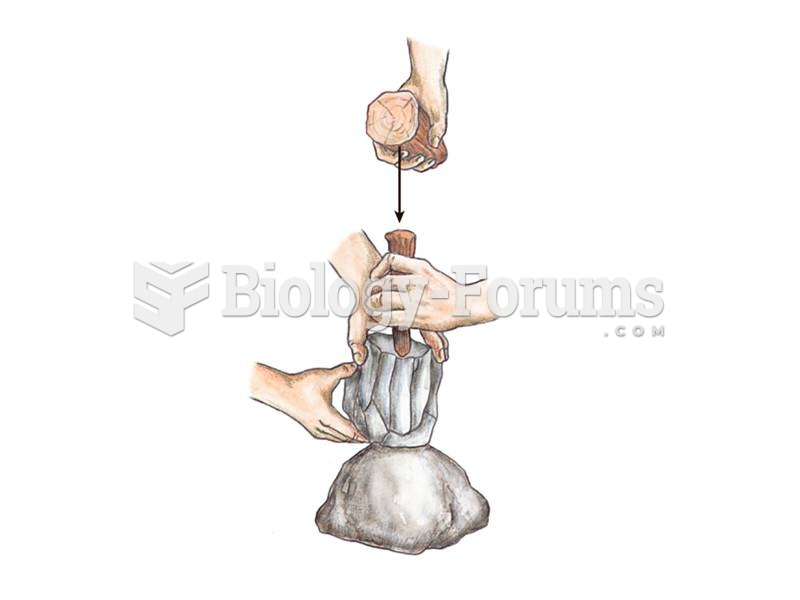
|
|
|
Less than one of every three adults with high LDL cholesterol has the condition under control. Only 48.1% with the condition are being treated for it.
For about 100 years, scientists thought that peptic ulcers were caused by stress, spicy food, and alcohol. Later, researchers added stomach acid to the list of causes and began treating ulcers with antacids. Now it is known that peptic ulcers are predominantly caused by Helicobacter pylori, a spiral-shaped bacterium that normally exist in the stomach.
The highest suicide rate in the United States is among people ages 65 years and older. Almost 15% of people in this age group commit suicide every year.
There are actually 60 minerals, 16 vitamins, 12 essential amino acids, and three essential fatty acids that your body needs every day.
The Babylonians wrote numbers in a system that used 60 as the base value rather than the number 10. They did not have a symbol for "zero."