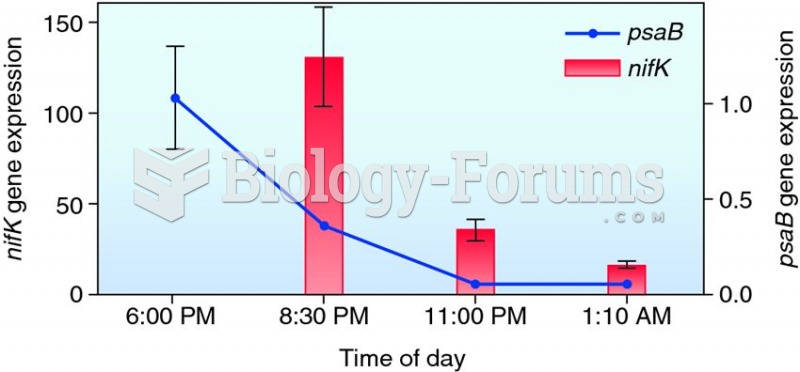
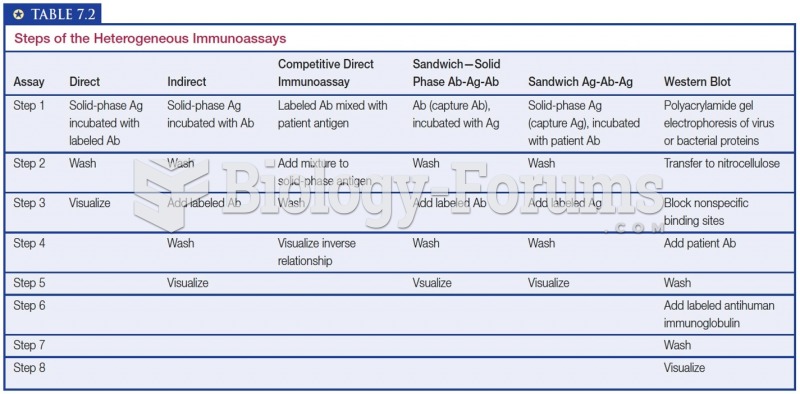
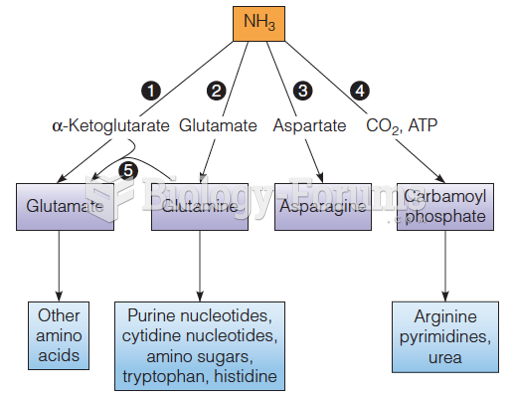
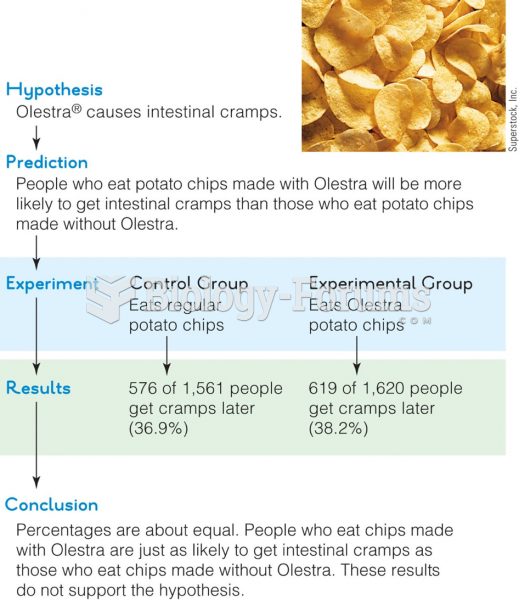
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The largest baby ever born weighed more than 23 pounds but died just 11 hours after his birth in 1879. The largest surviving baby was born in October 2009 in Sumatra, Indonesia, and weighed an astounding 19.2 pounds at birth.
Did you know?
There are 20 feet of blood vessels in each square inch of human skin.
Did you know?
People about to have surgery must tell their health care providers about all supplements they take.
Did you know?
Parkinson's disease is both chronic and progressive. This means that it persists over a long period of time and that its symptoms grow worse over time.
Did you know?
There are more nerve cells in one human brain than there are stars in the Milky Way.