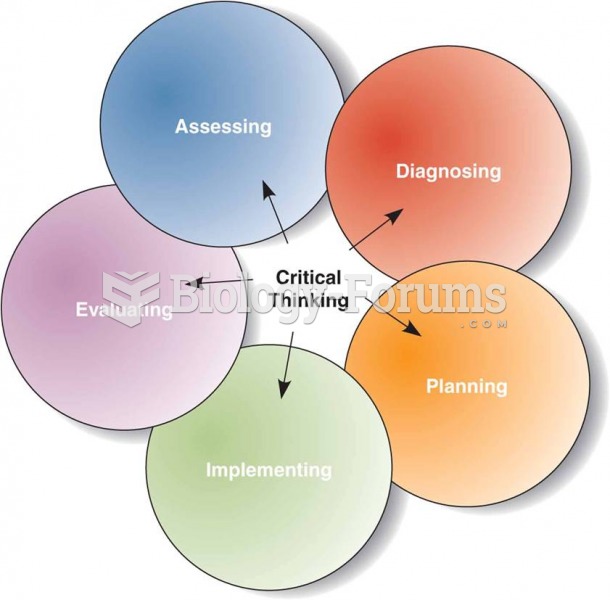
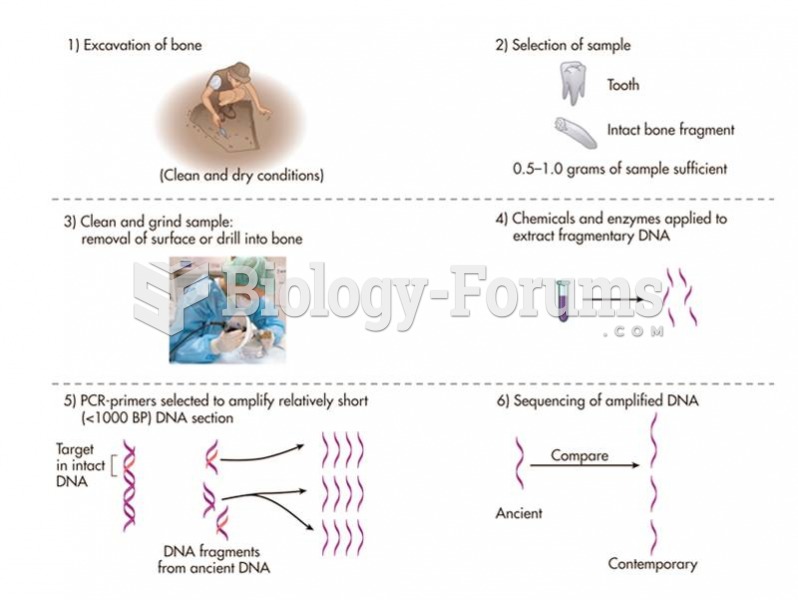
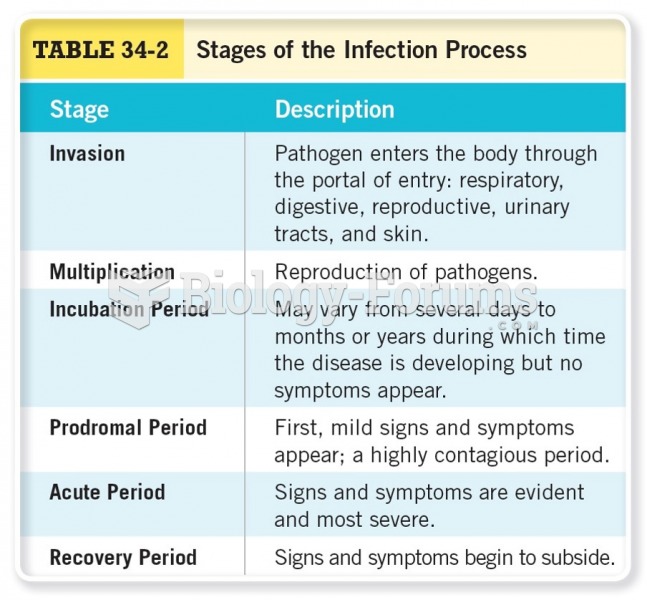
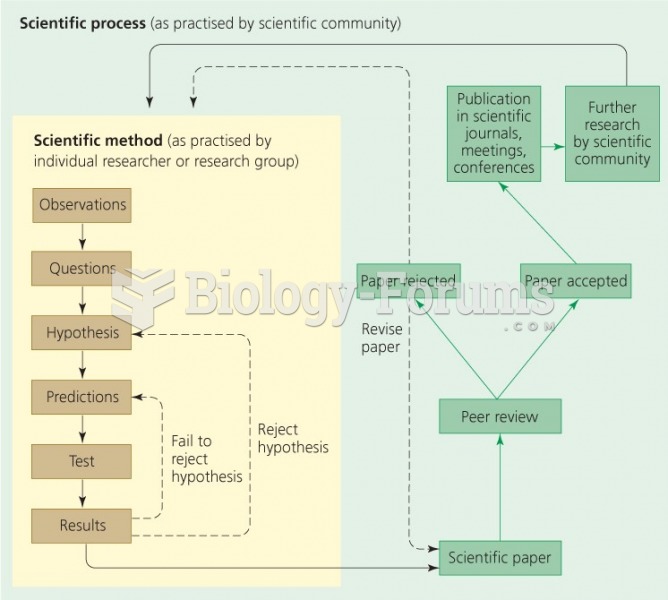
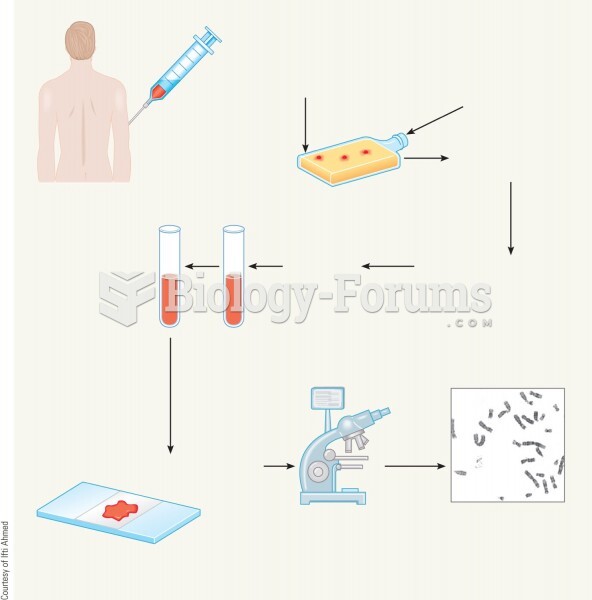
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The most common childhood diseases include croup, chickenpox, ear infections, flu, pneumonia, ringworm, respiratory syncytial virus, scabies, head lice, and asthma.
Did you know?
Fungal nail infections account for up to 30% of all skin infections. They affect 5% of the general population—mostly people over the age of 70.
Did you know?
Earwax has antimicrobial properties that reduce the viability of bacteria and fungus in the human ear.
Did you know?
More than 34,000 trademarked medication names and more than 10,000 generic medication names are in use in the United States.
Did you know?
In 1835 it was discovered that a disease of silkworms known as muscardine could be transferred from one silkworm to another, and was caused by a fungus.