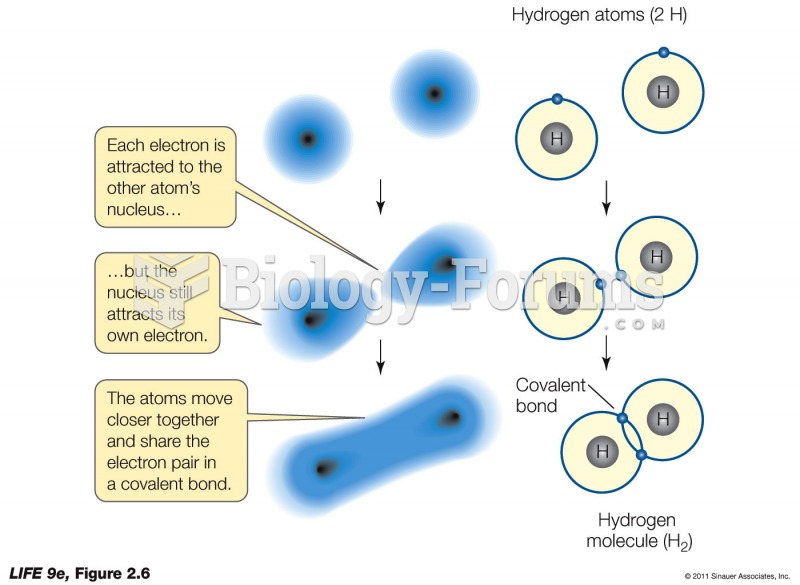
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
For pediatric patients, intravenous fluids are the most commonly cited products involved in medication errors that are reported to the USP.
Did you know?
There are approximately 3 million unintended pregnancies in the United States each year.
Did you know?
Complications of influenza include: bacterial pneumonia, ear and sinus infections, dehydration, and worsening of chronic conditions such as asthma, congestive heart failure, or diabetes.
Did you know?
Disorders that may affect pharmacodynamics include genetic mutations, malnutrition, thyrotoxicosis, myasthenia gravis, Parkinson's disease, and certain forms of insulin-resistant diabetes mellitus.
Did you know?
The heart is located in the center of the chest, with part of it tipped slightly so that it taps against the left side of the chest.