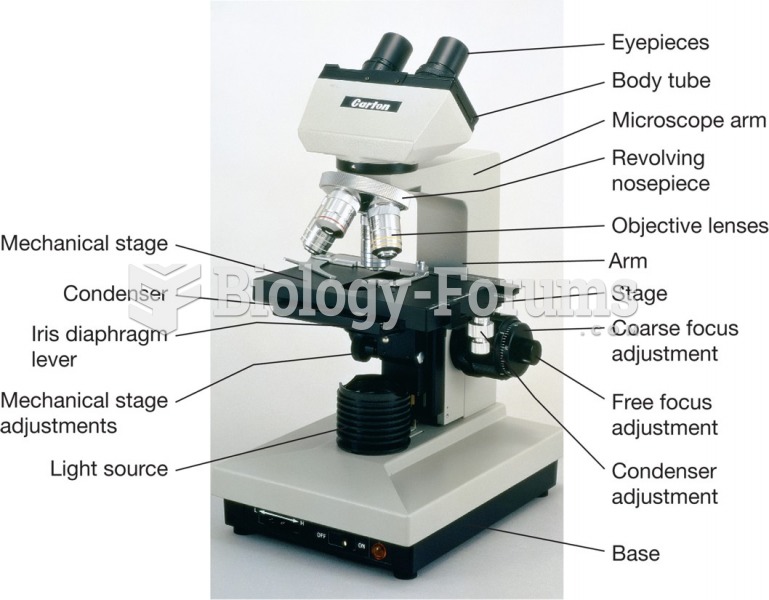
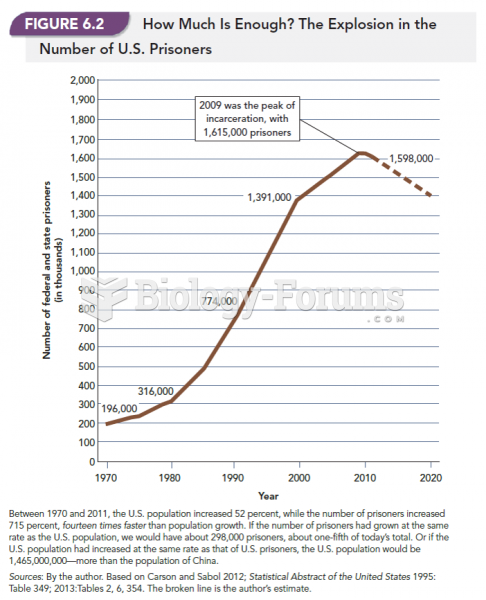
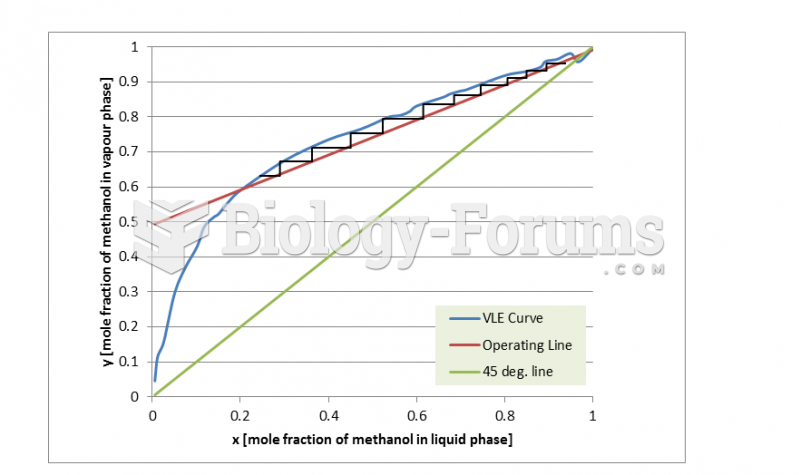
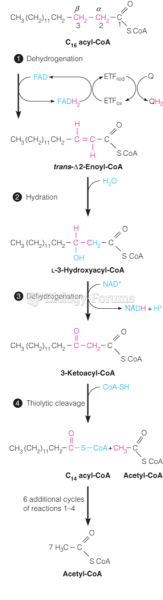
|
|
|
Eat fiber! A diet high in fiber can help lower cholesterol levels by as much as 10%.
Between 1999 and 2012, American adults with high total cholesterol decreased from 18.3% to 12.9%
The Romans did not use numerals to indicate fractions but instead used words to indicate parts of a whole.
Malaria mortality rates are falling. Increased malaria prevention and control measures have greatly improved these rates. Since 2000, malaria mortality rates have fallen globally by 60% among all age groups, and by 65% among children under age 5.
Asthma-like symptoms were first recorded about 3,500 years ago in Egypt. The first manuscript specifically written about asthma was in the year 1190, describing a condition characterized by sudden breathlessness. The treatments listed in this manuscript include chicken soup, herbs, and sexual abstinence.